Dose-dependent beneficial effect of melatonin on obesity; interaction of melatonin and leptin
Melatonin and obesity
Abstract
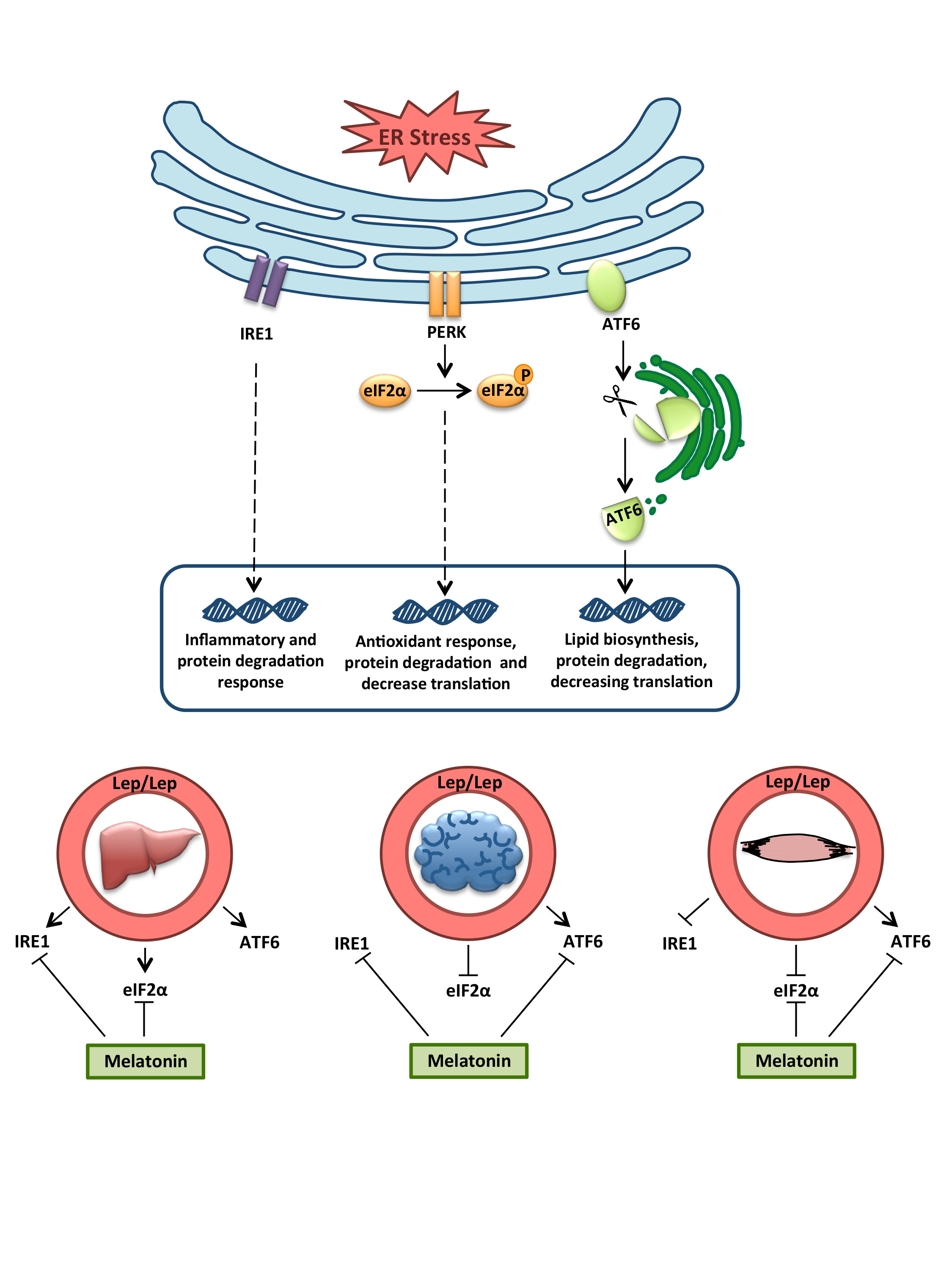
Although numerous studies have noted leptin’s role in obesity, there are still important mechanisms insights that need to be elucidated. Disturbed leptin production is associated with eating disorders, leading to alter food intake and energy expenditure. Proper regulation of protein homeostasis is critical for metabolic diseases such as obesity. Thus, the purpose of the present work was to study the unfolded protein response, which is implicated in the alleviation of endoplasmic reticulum stress-dependent dysregulation of nutritional status. We studied the effect of leptin deficiency on liver, brain and skeletal muscle tissues in obese (ob/ob) mice and the actions of a daily melatonin administration, as a possible treatment. Our findings showed that the leptin-deficient mice presented tissue-specific alterations of the three adaptive unfolded protein responses. ATF6α arm is strongly activated in all of them, indicating a deregulated lipid metabolism by the lack of leptin. Likewise, melatonin also alleviates unfolded protein response in a tissue-specific manner, acting mainly in the restoration of this disturbed ATF6α pathway. These findings support the use of melatonin as a potential therapeutic treatment against leptin-associated disorders.
References
2. Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, Robinson TN, Scott BJ, Jeor St, Williams CL (2005) Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation 111: 1999-2012.
3. Zieba DA, Szczesna M, Klocek-Gorka B, Williams GL (2008) Leptin as a nutritional signal regulating appetite and reproductive processes in seasonally-breeding ruminants. J. Physiol. Pharmacol. 59: Suppl 9, 7-18.
4. Cano P, Jimenez-Ortega V, Larrad A, Reyes Toso CF, Cardinali DP, Esquifino AI (2008) Effect of a high-fat diet on 24-h pattern of circulating levels of prolactin, luteinizing hormone, testosterone, corticosterone, thyroid-stimulating hormone and glucose, and pineal melatonin content, in rats. Endocrine 33: 118-125.
5. Mantele S, Otway DT, Middleton B, Bretschneider S, Wright J, Robertson MD, Skene J, Johnston JD (2012) Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PloS one 7: e37123.
6. Nguyen J, Wright KP, Jr (2010) Influence of weeks of circadian misalignment on leptin levels. Nat. Sci. Sleep 2: 9-18.
7. Buonfiglio D, Parthimos R, Dantas R, Cerqueira Silva R, Gomes G, Andrade-Silva J, Ramos-Lobo A, Amaral FG, Matos R, Sinesio j, Jr, Motta-Teixeira LJ, Donato J, Jr, Reiter RJ, Cipolla-Neto J (2018) Melatonin Absence Leads to Long-Term Leptin Resistance and Overweight in Rats. Front Endocrinol. 9: 122.
8. Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M, Soltani A (2017) Melatonin and human mitochondrial diseases. J. Res. Med. Sci. 2: 2.
9. Chakir I, Dumont S, Pevet P, Ouarour A, Challet E, Vuillez P (2015) Pineal melatonin is a circadian time-giver for leptin rhythm in Syrian hamsters. Front Neurosci. 9: 190.
10. Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257-1263.
11. Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, Rosales-Corral S, Tan DX, Reiter J (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71: 2997-3025.
12. Catania C, Binder E, Cota D (2011) mTORC1 signaling in energy balance and metabolic disease. Int. J. Obes. 35: 751-761.
13. Ali M, Bukhari SA, Ali M, Lee HW (2017) Upstream signalling of mTORC1 and its hyperactivation in type 2 diabetes (T2D). BMB Rep. 50: 601-609.
14. Kubrusly MS, Correa-Giannella ML, Bellodi-Privato M, de Sa SV, de Oliveira CP, Soares IC, Wakamatsu A, Alves VA, Giannella-Neto D, Bacchella T, Machado MC, D'Albuquerque LA (2010) A role for mammalian target of rapamycin (mTOR) pathway in non alcoholic steatohepatitis related-cirrhosis. Histol. Histopathol. 25: 1123-1131.
15. Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125: 443-451.
16. Yalcin A, Hotamisligil GS (2013) Impact of ER protein homeostasis on metabolism. Diabetes 62: 691-693.
17. Schroder M, Kaufman RJ (2005) ER stress and the unfolded protein response. Mutat. Res. 569: 29-63.
18. Gidalevitz T, Prahlad V, Morimoto RI (2011) The stress of protein misfolding: from single cells to multicellular organisms. Cold Spring Harb Perspect Biol. 3: 6.
19. Gotoh T, Endo M, Oike Y (2011) Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int. J. Inflam. 2011: 259462.
20. Thon M, Hosoi T, Yoshii M, Ozawa K (2014) Leptin induced GRP78 expression through the PI3K-mTOR pathway in neuronal cells. Sci. Rep. 4: 7096.
21. Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, Gentile CL (2016) Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism 65: 1238-1246.
22. Choi SI, Kim KS, Oh JY, Jin JY, Lee GH, Kim EK (2013) Melatonin induces autophagy via an mTOR-dependent pathway and enhances clearance of mutant-TGFBIp. J. Pineal Res. 54: 361-372.
23. Koh PO (2008) Melatonin prevents ischemic brain injury through activation of the mTOR/p70S6 kinase signaling pathway. Neurosci. Lett.. 444: 74-78.
24. Argiles JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Manas L (2016) Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 17: 789-796.
25. Yang L, Li P, Fu S, Calay ES, Hotamisligil GS (2010) Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11: 467-478.
26. de Luxán-Delgado B, Potes Y, Rubio-González A, Caballero B, Solano jj, Fernández-Fernández M, Bermúdez M, Guimaraes MRM, Vega-Naredo I, Boga JA, Coto-Montes A (2016). J. Pineal. Res. 61: 108-123.
27. Flamment M, Hajduch E, Ferre P, Foufelle F (2012) New insights into ER stress-induced insulin resistance. Trends Endocrinol. Metab. 23: 381-390.
28. Cota D, Matter EK, Woods SC, Seeley RJ (2008) The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J. Neurosci. 28:7202-7208.
29. Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW (2009) ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J. Cell Sci. 122: 1626-1636.
30. Rubio-Gonzalez A, Bermejo-Millo JC, de Luxan-Delgado B, Potes Y, Perez-Martinez Z, Boga JA, Vega-Naredo I, Caballero B, Solano JJ, Coto-Montes A (2018) Melatonin Prevents the Harmful Effects of Obesity on the Brain, Including at the Behavioral Level. Mol. Neurobiol. 55: 5830-5846.
31. Lipina C, Hundal HS (2017) Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle. 8: 190-201.
32. Carbone JW, McClung JP, Pasiakos SM (2012) Skeletal muscle responses to negative energy balance: effects of dietary protein. Ad. Nutr. 3: 119-126.
33. Stacchiotti A, Favero G, Giugno L, Golic I, Korac A, Rezzani R. (2017) Melatonin Efficacy in Obese Leptin-Deficient Mice Heart. Nutrients 9: 1323.
34. Szewczyk-Golec K, Wozniak A, Reiter RJ. (2015) Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J. Pineal Res. 59: 277-291.
35. Jimenez-Aranda A, Fernández-Vázquez G, Campos D, Tassi M, Velasco-Pérez L, Tan DX, Reiter RJ, Agil A. (2013) Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 55: 416-423.
36. Coto-Montes A, Boga JA, Tan DX, Reiter RJ. (2016) I Melatonin as a Potential Agent in the Treatment of Sarcopenia. Int. J. Mol. Sci. 17: 1771.
37. Brodsky VY, Zvezdina ND. (2010) Melatonin as the most effective organizer of the rhythm of protein synthesis in hepatocytes in vitro and in vivo. Cell Biol. Int. 34: 1199-1204.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


