Plant Perception of Light: The role of indoleamines in Scutellaria species
Perception of light: melatonin’s favourite colours
Abstract
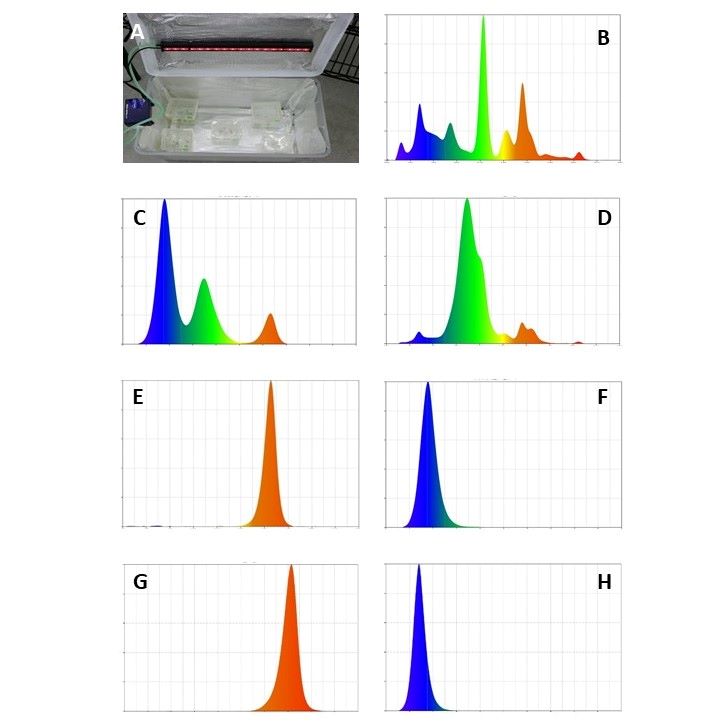
Light mediates plant growth through diverse mechanisms and signaling networks including plant growth regulators (PGRs). We hypothesized that a novel class of PGRs, the indoleamines, are plant signaling molecules that perceive changes in light composition and initiate a cascade of metabolic responses. We used three Scutellaria model species (skullcap): S. lateriflora, S. galericulata and S. racemosa that produce high levels of melatonin and serotonin to investigate this hypothesis. Axenic Scutellaria cultures were exposed to red, blue, green or full spectrum white light spectra provided by light emitting diode (LED) lighting systems, or daylight fluorescent bulbs. Melatonin (MEL), serotonin (5HT), abscisic acid (ABA), auxin (IAA), and jasmonic acid (JA), were quantified by liquid chromatography with tandem mass spectrometry. Melatonin was detected consistently in plants grown under blue light in all species of Scutellaria. In S. galericulata, significant quantities of ABA were detected in plants grown under white light but not detected in plants grown under other light spectra. In timeline studies of S. racemosa plants exposed to limited red or blue light spectra had significantly reduced levels of tryptamine (TRM), 5HT and MEL in the shoots initially but melatonin was detected after 12 hours and quantifiable amounts of 5HT were detected after 7 days. Supplementation of the culture medium with MEL or 5HT did not change the pattern of MEL in blue light grown cultures but did change patterns of 5HT accumulation. 5HT was highest in plants grown under red light immediately after culture and decreased over 7 days. These data indicate that the relative amounts of MEL and 5HT are responsive to light spectra and redirect metabolic resources to enable plant adaptations to changing environments.
References
2. Cole IB, Saxena PK, Murch SJ (2007) Medicinal biotechnology in the genus Scutellaria. Vitr. Cell Dev. Biol. Plant 43: 318–327. https://doi.org/10.1007/s11627-007-9055-4.
3. Cole IB, Cao J, Alan AR, et al. (2008) Comparisons of Scutellaria baicalensis, Scutellaria lateriflora and Scutellaria racemosa: Genome size, antioxidant potential and phytochemistry. Planta Med. 74: 474–481. https://doi.org/10.1055/s-2008-1034358.
4. Bianchi A (2006) Attivita antidepressiva di due specie di Scutellariae colombiane. In: Annual Meeting for the Instruction of Acupuncture and Reflexology - Torino.
5. Xiao K, Han Q-T, Zhang L, Dai S (2017) Two new flavanone glycosides from Scutellaria galericulata with anti- inflammatory activities. Phytochem. Lett. 20: 151–154.
6. He CN, Peng Y, Xiao W, Xiao PG (2012) The ethnopharmacological investigation of Chinese Scutellaria plants. Mod. Chin. Med. 14: 16–20.
7. Murch SJ, Simmons CB, Saxena PK (1997) Melatonin in feverfew and other medicinal plants. Lancet 350: 1598–1599.
8. Posmyk MM, Balabusta M, Wieczorek M, et al. (2009) Melatonin applied to cucumber (Cucumis sativus L .) seeds improves germination during chilling stress. J. Pineal. Res. 46: 214–223. https://doi.org/10.1111/j.1600-079X.2008.00652.x.
9. Erland LAE, Murch SJ, Reiter RJ, Saxena PK (2015) A new balancing act: The many roles of melatonin and serotonin in plant growth and development. Plant. Signal. Behav. Behav. 10: e1096469. https://doi.org/10.1080/15592324.2015.1096469.
10. Erland LAE, Saxena PK (2018) Melatonin in plant morphogenesis. Vitr. Cell. Dev. Biol. Plant 54: 3–24. https://doi.org/10.1007/s11627-017-9879-5.
11. Murch SJ, KrishnaRaj S, Saxena PK (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 19: 698–704. https://doi.org/10.1007/s002990000206.
12. Murch SJ, Campbell SSB, Saxena PK (2001) The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John’s wort (Hypericum perforatum L.). Vitr. Cell. Dev. Biol. Plant 37: 786–793.
13. Kim M, Seo H, Park C, Park WJ (2016) Examination of the auxin hypothesis of phytomelatonin action in classical auxin assay systems in maize. J. Plant. Physiol. 190: 67–71. https://doi.org/10.1016/j.jplph.2015.11.009.
14. Arnao MB, Hernández-Ruiz J (2017) Growth activity, rooting capacity, and tropism: three auxinic precepts fulfilled by melatonin. Acta. Physiol. Plant 39: 1–9. https://doi.org/10.1007/s11738-017-2428-33.
15. Erland LAE, Saxena PK, Murch SJ (2018) Melatonin in plant signalling and behaviour. Funct. Plant Biol. 45: 58–69. https://doi.org/10.1071/FP16384.
16. Wei J, Li DX, Zhang JR, et al. (2018) Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal. Res. 65: 1–13. https://doi.org/10.1111/jpi.12500.
17. Sliwiak J, Dauter Z, Jaskolski M (2016) Crystal structure of Hyp-1, a Hypericum perforatum PR-10 protein, in complex with melatonin. Front. Plant Sci. 7: 1–10. https://doi.org/10.3389/fpls.2016.00668.
18. Sliwiak J, Sikorski M, Jaskolski M (2018) PR-10 proteins as potential mediators of melatonin- cytokinin cross-talk in plants : crystallographic studies of LlPR-10.2B isoform from yellow lupine. FEBS J. 285: 1907–1922. https://doi.org/10.1111/febs.14455.
19. Sherif SM, Shukla MR, Murch SJ, et al. (2016) Simultaneous induction of jasmonic acid and disease-responsive genes signifies tolerance of American elm to Dutch elm disease. Sci. Rep. 6: 1–15. https://doi.org/10.1038/srep21934.
20. Saremba BM, Murch SJ, Tymm FJM, Rheault MR (2018) The metabolic fate of dietary nicotine in the cabbage looper, Trichoplusia ni (Hübner). J. Insect Physiol. 109: 1–10. https://doi.org/10.1016/j.jinsphys.2018.05.010.
21. Arnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress ? Trends Plant Sci. 19: 789–797. https://doi.org/10.1016/j.tplants.2014.07.006.
22. Murch SJ (2006) Neurotransmitters, neuroregulators and neurotoxins in plants. Communication in Plants, eds Baluška F., Mancuso S., Volkmann D. (Springer, Heidelberg), pp. 137–151. https://doi.org/10.1007/978-3-540-28516-8_10.
23. Afreen F, Zobayed SMA, Kozai T (2006) Melatonin in Glycyrrhiza uralensis: Response of plant roots to spectral quality of light and UV-B radiation. J. Pineal. Res. 41: 108–115. https://doi.org/10.1111/j.1600-079X.2006.00337.x.
24. Hernández-Ruiz J, Arnao MB (2008) Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Regul. 55: 29–34. https://doi.org/10.1007/s10725-008-9254-y.
25. Li H, Murch SJ, Saxena PK (2000) Thidiazuron-induced de novo shoot organogenesis on seedlings, etiolated hypocotyls and stem segments of Huang-qin. Plant Cell Tissue Organ Cult. 62: 169–173.
26. Murashige, T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 15: 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x.
27. Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell. Res. 50: 151–158. https://doi.org/10.1016/0014-4827(68)90403-5.
28. Lazár D, Murch SJ, Beilby MJ, Al Khazaaly S (2013) Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal Behav. 8: 1–5. https://doi.org/10.4161/psb.23279.
29. Beilby MJ, Turi CE, Baker TC, et al. (2015) Circadian changes in endogenous concentrations of indole-3-acetic acid, melatonin, serotonin, abscisic acid and jasmonic acid in Characeae (Chara australis brown). Plant Signal Behav. 10: e1082697. https://doi.org/10.1080/15592324.2015.1082697.
30. Saremba BM, Tymm FJM, Baethke K, et al. (2017) Plant signals during beetle (Scolytus multistriatus) feeding in American elm (Ulmus americana Planch). Plant Signal Behav. 12: e1296997. https://doi.org/10.1080/15592324.2017.1296997.
31. Pelagio-Flores R, Muñoz-Parra E, Ortiz-Castro R, López-Bucio J (2012) Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal. Res. 53: 279–288. https://doi.org/10.1111/j.1600-079X.2012.00996.x.
32. Sanchez-Barcelo EJ, Mediavilla MD, Vriend J, Reiter RJ (2016) Constitutive photomorphogenesis protein 1 (COP1) and COP9 signalosome, evolutionarily conserved photomorphogenic proteins as possible targets of melatonin. J. Pineal. Res. 1: 41–51. https://doi.org/10.1111/jpi.12340.
33. Steward FC, Kent AE, Mapes MO (1967) Growth and organization in cultured cells: Sequential and synergistic effects of growth-regulating substances.. Ann. N. Y. Acad. Sci. 144: 326–334. https://doi.org/10.1111/j.1749-6632.1967.tb34028.x.
34. Murch SJ, Saxena PK (2002) Melatonin: A potential regulator of plant growth and development? Vitr. Cell Dev. Biol. Plant 38: 531–536. https://doi.org/10.1079/IVP2002333.
35. Stutte GW (2015) Commercial transition to leds: A pathway to high-value products. HortScience. 50: 1297–1300.
36. Brazaityte A, Sakalauskiene S, Samuoliene G, et al. (2015) The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 173: 600–606. https://doi.org/10.1016/j.foodchem.2014.10.077.
37. Długosz-Grochowska O, Wojciechowska R, Kruczek M, Habela A (2017) Supplemental lighting with LEDs improves the biochemical composition of two Valerianella locusta (L.) cultivars. Hortic. Environ. Biotechnol. 58: 441–449. https://doi.org/10.1007/s13580-017-0300-4.
38. Zhang Y, Jiang L, Li Y, et al. (2018) Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa). Molecules 23: 1–17. https://doi.org/10.3390/molecules23040820.
39. Zoratti L, Sarala M, Carvalho E, et al. (2014) Monochromatic light increases anthocyanin content during fruit development in bilberry. BMC. Plant Biol. 14: 1–10. https://doi.org/10.1186/s12870-014-0377-1.
40. Zhang N, Sun Q, Li H, et al. (2016) Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 7: 1–17. https://doi.org/10.3389/fpls.2016.00197.
41. Khan T, Ullah MA, Garros L, et al. (2019) Synergistic effects of melatonin and distinct spectral lights for enhanced production of anti-cancerous compounds in callus cultures of Fagonia indica. J. Photochem. Photobiol. B. Biol. 190: 163–171.
42. West KE, Jablonski MR, Warfield B, et al. (2011) Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J. Appl. Physiol. 110: 619–626. https://doi.org/10.1152/japplphysiol.01413.2009.
43. Kolár J, Johnson CH, Machácková I (2002) Presence and possible role of melatonin in a short-day flowering plant, Chenopodium rubrum. Melatonin After Four Decades. pp 391–393.
44. Wolf K, Kolář J, Witters E, et al. (2001) Daily profile of melatonin levels in Chenopodium rubrum L. depends on photoperiod. J. Plant Physiol. 158: 1491–1293.
45. Hardeland R (2015) Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J. Exp. Bot. 66: 627–646. https://doi.org/10.1093/jxb/eru386.
46. Chen Z, Xie Y, Gu Q, et al. (2017) The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radic. Biol. Med. 108: 465–477. https://doi.org/10.1016/j.freeradbiomed.2017.04.009.
47. Morre D, Morre D (2003) The plasma membrane-associated NADH oxidase (ECTO-NOX) of mouse skin responds to blue light. J. Photochem. Photobiol. B. Biol. 70: 7–12. https://doi.org/10.1016/S1011-1344(03)00023-X.
48. Das R, Sopory SK (1985) Evidence of Regulation of Calcium Uptake By Phytochrome in Maize Protoplasts. Biochem. Biophys. Res. Commun. 128: 1455–1460.
49. Huang Y, Kao CH (1992) Calcium in the regulation of corn leaf senescence by light. Bot. Bull. Acad. Sin. 33: 161–165.
50. Chandok MR, Sopory SK (1994) 5-Hydroxytryptamine affects turnover of polyphosphoinositides in maize and stimulates nitrate reductase in the absence of light. FEBS Lett. 356: 39–42. https://doi.org/10.1016/0014-5793(94)01213-X.
51. Erland LAE, Turi CE, Saxena PK (2016) Serotonin: An ancient molecule and an important regulator of plant processes. Biotechnol. Adv. 34: 1347–1361. https://doi.org/10.1016/j.biotechadv.2016.10.002.
52. Neff MM (2012) Light-mediated seed germination: connecting phytochrome b to gibberellic acid. Dev. Cell 22: 687–688. https://doi.org/10.1016/j.devcel.2012.04.003.
53. Li C, Tan D, Liang D, et al. (2015) Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 66: 669–680. https://doi.org/10.1093/jxb/eru476.
54. Ishihara A, Hashimoto Y, Tanaka C, et al. (2008) The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 54: 481–495. https://doi.org/10.1111/j.1365-313X.2008.03441.x.
55. Sauer M, Robert S, Kleine-Vehn J (2013) Auxin: simply complicated. J. Exp. Bot. 64: 2565–2577. https://doi.org/http://doi.org/10.1093/jxb/ert139.
56. Heitz T, Smirnova E, Marquis V, Poirier L (2019) Metabolic Control within the Jasmonate Biochemical Pathway. Plant Cell Physiol. 60: 2621–2628. https://doi.org/10.1093/pcp/pcz172.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


