Please cite this paper as:
Wongchitrat, P., Yasawong, M., Jumpathong, W., Chanmanee, T., Samutpong, A., Dangsakul, W., Govitrapong, P., Reiter, R.J. and Puthavathana, P. 2022. Melatonin inhibits Zika virus replication in Vero epithelial cells and SK-N-SH human neuroblastoma cells. Melatonin Research. 5, 2 (Jun. 2022), 171-185. DOI:https://doi.org/https://doi.org/10.32794/mr112500127.
Research Article
Melatonin inhibits Zika virus replication in Vero epithelial cells and SK-N-SH human neuroblastoma cells
Prapimpun Wongchitrat*1, , Montri Yasawong2, Watthanachai Jumpathong3, Tipsuda Chanmanee1, Arisara Samutpong1, Worawat Dangsakul1,4, Piyarat Govitrapong5, Russel J. Reiter6, Pilaipan Puthavathana*1,
1Center for Research and Innovation, Faculty of Medical Technology, Mahidol University, Salaya, Nakon Pathom, 73170, Thailand
2Program on Environmental Toxicology, Chulabhorn Graduate Institute, Chulabhorn Royal Academy, Bangkok, 10210, Thailand
3Program on Chemical Sciences, Chulabhorn Graduate Institute, Chulabhorn Royal Academy, Bangkok, 10210, Thailand
4Regional Medical Sciences Center 5th Samut Songkhram, Samut Songkhram, 75110, Thailand
5Chulabhorn Graduate Institute, Chulabhorn Royal Academy, Bangkok, 10210, Thailand
6Department of Cell Systems & Anatomy, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA
*Correspondence: prapimpun.won@mahidol.ac.th, pilaipan.put@mahidol.edu, Tel: +66(2)441 4371, Fax: +66(2)441 4380
Running title: Inhibitory effect of melatonin on Zika virus replication
Received: April 20, 2022; Accepted: June 18, 2022
ABSTRACT
Zika virus (ZIKV) is an emerging flavivirus that is causing significant public health concerns worldwide because of its association with severe neurological disorders in both newborns and adults. At present, the search for effective antiviral drugs for ZIKV infection is intense. Melatonin is a molecule that influences a wide variety of physiological processes. Melatonin is a direct free radical scavenger, an indirect antioxidant due to its stimulation of antioxidant enzymes and an anti-inflammatory and immunoregulatory molecule; recent studies also have shown melatonin to have anti-viral activity. Here, we are the first to show that melatonin inhibits ZIKV replication in both Vero cells and SK-N-SH cells. The suppressive actions of melatonin pretreatment on ZIKV infection was found to be time- and dose-dependent. The inhibitory effect of melatonin was more pronounced in human SK-N-SH neural cells than in Vero cells. Molecular docking experiments showed that melatonin exhibited high binding affinity to ZIKV nonstructural 3 (NS3) protein, the possible underlying inhibitory mechanism of melatonin on ZIKV replication. The results suggest that melatonin may have potential prophylactic and treatment effects against ZIKV infection.
Key words: Melatonin; Zika virus; flavivirus; viral replication; mechanisms of action; antiviral activity; Vero epithelial cells; SK-N-SH human neuroblastoma cells
_____________________________________________________________________________
1. INTRODUCTION
Zika virus (ZIKV) is a mosquito-borne virus belonging to the genus Flavivirus (family Flaviviridae) which includes other members such as dengue virus (DENV), Japanese encephalitis virus (JEV), yellow fever virus (YFV) and West Nile virus (WNV). ZIKV infection is usually asymptomatic or causes mild self-limiting symptoms of a dengue-like illness (low-grade fever, headache, rash, conjunctivitis, arthritis and myalgia) (1). ZIKV was recognized as an emerging health problem when it caused an outbreak in Yap Island, French Polynesia in 2013 (2). An uncommonly severe complication associated with a ZIKV infection, Guillain-Barre syndrome, was first reported during this outbreak (3-5). In 2016, World Health Organization (WHO) declared ZIKV infection a major public health concern because of an unusual increase in the number of congenital microcephalic newborns, a consequence of ZIKV infection in pregnant women during early gestation (6). Unfortunately, neither an effective vaccine nor a prophylactic or therapeutic treatment against ZIKV exists to control the future outbreaks.
ZIKVs are enveloped, spherical particles 40–60 nm in diameter (7). The virion contains a positive sense, single-stranded RNA genome of 10.7 kilobases in length. The viral genome encodes 3 structural proteins, i.e., capsid, precursor membrane, and envelope, together with seven nonstructural proteins (NS) i.e., NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 (8). ZIKV enters cells via the mechanism of receptor-mediated endocytosis, replicates in the cytoplasm within insect and human hosts and is then released from the cytoplasmic membrane. ZIKV can be found in numerous body fluids including blood, saliva, urine, breast milk, cerebrospinal fluid, semen and amniotic fluid (9, 10). Crucial concerns about ZIKV infection have arisen particularly considering the neurological damage in maturating fetuses. Since ZIXV infections currently lack treatment, the development of effective and potent antivirals against ZIKV is urgently needed.
Melatonin (N-[2-(5-methoxy-1H-indol-3-yl)ethyl]) (Figure 1A), is a secretory product of the pineal gland of vertebrates from which it is released into the blood and cerebrospinal fluid (CSF) (11, 12) in a cyclic manner under the control of circadian biological clock, the suprachiasmatic nucleus in the basal hypothalamus (13). Additionally, melatonin is produced in many other organs from which it is not released into the vascular system (14). The circadian rhythm of melatonin in the blood and CSF provides chronobiotic information that synchronizes and stabilizes bodily physiological and behavioral rhythms such as the sleep/wake cycle (15). As to its biological modulatory actions, melatonin functions as strong free radical scavenger (16) and anti-inflammatory agent (17) and has inhibitory actions against several pathogens including bacteria, parasites and viruses (18-21). The antiviral activity of melatonin both in vitro and in vivo was previously observed on several RNA viruses belonging to families Togaviridae, Orthomyxoviridae, Caliciviridae, Flaviviridae and Coronaviridae (22-25). The antiviral properties of melatonin against these viruses may rely, in part, on its potent antioxidant, anti-inflammatory, immunoregulatory, and cellular protective effects (20, 24, 26, 27). Considering that melatonin has demonstrated antiviral activity together with the need for a drug to combat the ZIKV, we investigated the potential antiviral effect of melatonin in Vero cells and SK-N-SH human neuronal cells infected with the ZIKV.
2. MATERIALS AND METHODS
2.1. Cell lines.
The Vero epithelial cell line (ATCC No. CCL-81) (originally derived from the African green monkey kidney cell line), and the SK-N-SH human neuroblastoma cell line (ATCC No. HTB-11) were used in this study. Vero and SK-N-SH cells were grown in Eagle’s Minimum Essential Medium (EMEM) (Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco), amphotericin B, and antibiotics, and maintained in 2% FBS in a 5% CO2 incubator incubated at 37 °C in a humidified chamber.
2.2. Virus propagation.
ZIKV strain MUMT-1/2016 (NCBI accession no: MT377392) of the Asian lineage was isolated and propagated in Aedes albopictus C6/36 cell lines (ATCC No. CRL-1660) and maintained in in Leibovitz's L-15 Medium (Gibco) supplemented with 5% FBS at 28 °C for approximately 6 days. The culture supernatants were pooled, aliquoted, and stored at -80 °C until use.
2.3 Foci-forming unit (FFU) assay.
Viral titration was performed by FFU assay in Vero cell monolayers in 96-well plates. The virus suspension was serially diluted 10-fold and a volume of 50 μL of each dilution was inoculated onto Vero cell monolayers. After virus absorption for 2 h, the inoculum was removed, overlaid with culture medium containing 3% FBS and 1.5% carboxymethyl cellulose, and incubated at 37 °C in a CO2 incubator for 3 days. Then, the reaction plates were stained with the 4G2 monoclonal antibody (Millipore Sigma, Burlington, MA, USA) targeting the flaviviral envelope protein, followed by goat anti-mouse immunoglobulin conjugated with horseradish peroxidase (Millipore Sigma). In addition, 3,3′-diaminobenzidine was used as a chromogenic substrate. The dark brown foci of the virus infected cells were counted and expressed as foci-forming unit (FFUs)/mL.
2.4. Cytotoxicity assay.
Melatonin of analytical grade (Sigma-Aldrich, St. Louis, MO, USA) was used in this study. Melatonin solution was freshly prepared and protected from light during usage. Melatonin was dissolved in a small volume of 40% ethanol to yield a stock of 10 mM solution and then further serially diluted in culture medium with 2% FBS to obtain the desired concentration. The cytotoxicity of melatonin to Vero cells and SK-N-SH cells was investigated using the MTS method using CellTiter 96® AQueous One Solution Cell Proliferation (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The percentage of cell viability was calculated relative to control cells designated as 100% viable cells. The 50% cytotoxic concentration (CC50) of melatonin was defined as the concentration that reduced half of the cells, was calculated.
2.5. Antiviral activity experiment.
Melatonin was tested for its antiviral activity against ZIKV infection. Vero cells or SK-N-SH cells were seeded on 96-well plates to reach 80-90% confluence in 24 h at 37 °C in 5% CO2. Cells were pretreated with melatonin for 1 h. After that, cells were infected with ZIKV at multiplicity of infection (MOI) of 0.1 after melatonin was removed. Then, the infection mixtures were removed and washed with culture medium to eliminate the excess virus. The infected cells were further incubated in medium containing the indicated concentration of melatonin during the treatment periods. A schematic of the treatment procedure is shown in Figure 1B. Supernatants from each treatment were collected 1, 2 and 3 days after infection. ZIKV-induced cytopathicity (CPE) was observed and photographed under a microscope. The antiviral effect of melatonin on virus yields in cell culture supernatant was determined by a FFU assay in both types of cell lines. The concentration that results in a 50% inhibitory effect (IC50) of melatonin, which is defined as the concentration that inhibits half of ZIKV infection, was calculated.
2.6. Molecular docking.
Molecular docking analysis was performed to analyze and to predict potential inhibitory activities against ZIKV proteins related to viral genome replication, including NS1, NS2B-NS3, NS3 and NS5. The binding properties of the ligand to each protein were analyzed using AutoDock Vina. 1.1.2 software (28). Molecular docking of melatonin with each ZIKV protein of interest was performed in which grid boxes were constructed according to the parameters tabulated in supplementary Table 1, where quinone reductase 2 (QR2) was used as a reference protein that shows the interaction with melatonin by molecular docking analysis (29, 30). One thousand exhaustive searches were performed for molecular docking. The 2D ligand-protein interaction diagrams of melatonin and ZIKV protein were constructed using LigPlot+ 2.2 software (31).
2.7. Statistical analysis.
The cell viability values and virus titers were fit into nonlinear regression curves and the CC50 and IC50 values of melatonin on each type of cell were then calculated respectively, using GraphPad Prism (v6.0, GraphPad software Inc., San Diego, CA, USA) by interpolation. The data are expressed as the mean ± standard error of the mean (SEM) from three independent experiments performed in triplicate. Significant differences were tested using one- or two-way analysis of variance (ANOVA) to determine the effect of dose and time; subsequently, post hoc multiple analysis was performed as appropriate. Values of P < 0.05 were considered significant.
3. RESULTS
3.1. Cytotoxicity of melatonin.
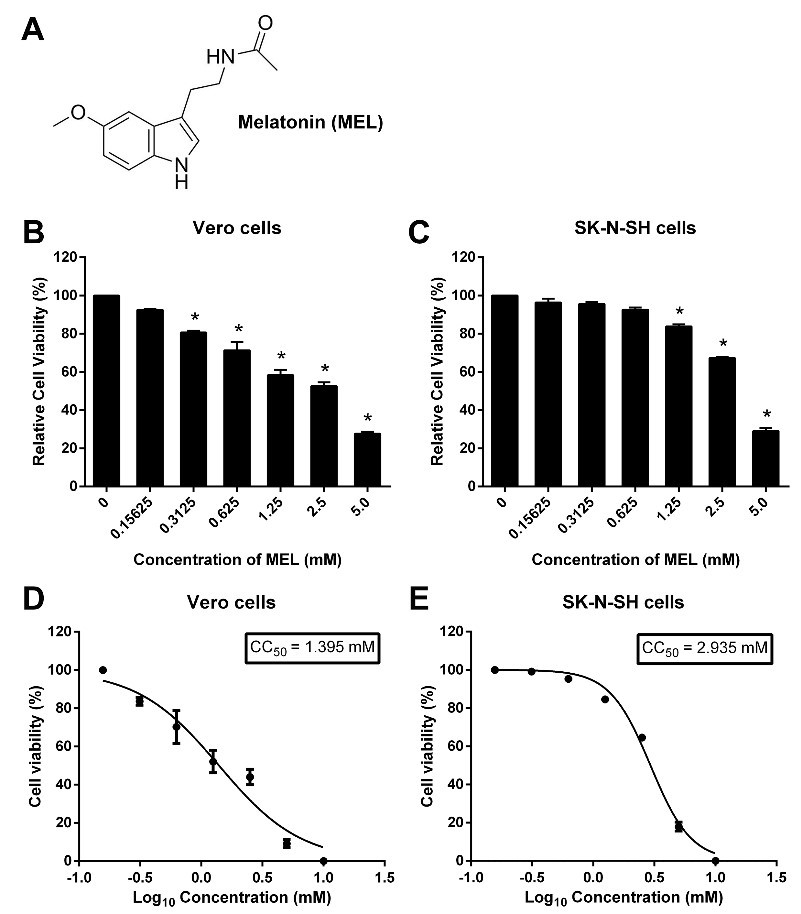
To determine the cytotoxic effect of melatonin for further study on its antiviral activity in the tested cell lines, different concentrations of melatonin were investigated using the MTS assay. The cytotoxic effects of melatonin on Vero cells (Figure 1B) and SK-N-SH cells (Figure 1C) were demonstrated at 24 h posttreatment which corresponded well to the morphological changes and the decreased survival of both cell lines. The CC50 of melatonin was 1.395 mM for Vero cells (Figure 1D) and 2.935 mM for SK-N-SH cells (Figure 1E). When cells were treated with vehicle control, ethanol did not show any cytotoxicity against either tested cell lines. Therefore, melatonin concentration below CC50 values of 0.25, 0.5 and 1 mM for Vero cells and 0.15, 0.3, 0.6 and 1.2 mM for SK-N-SH cells were selected for subsequent antiviral assay experiments.

Fig. 1. Citotoxicity test of melatonin.
A. Chemical structure of melatonin (MEL). B. Viability of Vero cells. C. Viability of SK-N-SH cells. Cells were treated with different concentrations of melatonin. Melatonin was serially diluted to concentrations of 0.15625, 0.3125, 0.625, 1.25, 2.5 and 5 mM. Subsequently, the cytotoxicity of melatonin was determined by MTS assay. After 24 h of incubation, the optical density of the reaction was measured at a wavelength of 490 nm. The percentage of cell viability is presented compared to the cell control. The results are indicated as the mean ± SEM of three independent experiments. One-way ANOVA and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05 compared with control. The CC50 values of melatonin for D) Vero and E) SK-N-SH were calculated using GraphPad Prism by interpolation.
3.2. Melatonin pre- and post-treatment reduces the release of infectious ZIKV progeny.
To study whether melatonin had an antiviral effect on ZIKV propagation, cells were pre-treated with melatonin 1 h before virus exposure. Subsequently, the infected cells were treated with or without various concentration of MEL for 1, 2 and 3 dpi. The inhibition of viral production in culture supernatant was determined by foci forming reduction assay.
In the Vero cells (Figure 2B and 2C), the melatonin treatment of ZIKV-infected cells with 0.25 mM showed the lowest effect on the reduction in the virus yield up to ~35% compared to other concentrations at 1 dpi, while 0.25 mM melatonin failed to suppress viral production as shown at 3 dpi. A medium to high display of an ~50-70% reduction in the virus yield was observed at 0.5 and 1 mM melatonin treatment only at 1 dpi. A significant inhibitory effect of melatonin at both 0.5 and 1 mM was observed at all time points (P < 0.05). However, the reduction in the virus yield gradually decreased at 2 and 3 dpi for all doses of melatonin treatment in ZIKV-infected cells compared to the effect at 1 dpi (P < 0.05). The 50% inhibitory effect (IC50) of melatonin in the Vero cells, was determined to be 0.2477, 0.3300 and 0.4110 mM for days 1, 2 and 3 of ZIKV infection, respectively.
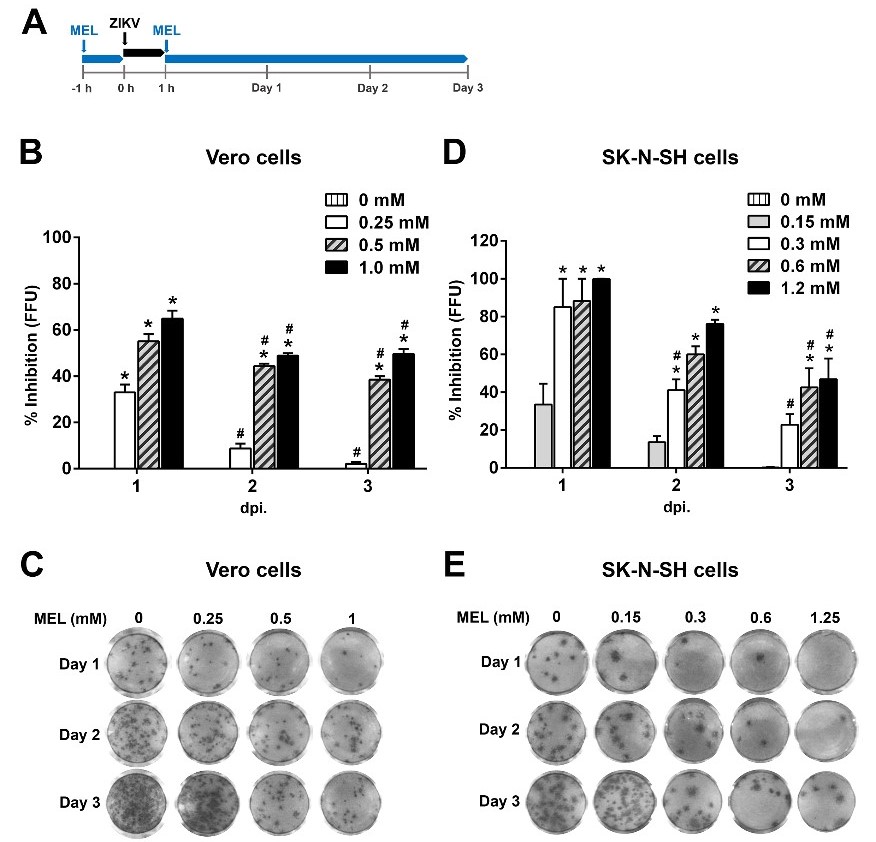
Fig. 2. Inhibitory effect of melatonin (MEL) on ZIKV yields.
A. The schematic of virus infection and MEL treatment regimens. B, C. Vero cells. D, E. SK-N-SH Cells. The cells were pretreated with melatonin and then infected with ZIKV at MOI of 0.1, and post-treated with melatonin for 1, 2 and 3 days. Progeny viruses in cell culture supernatants were measured by a foci forming unit reduction assay to evaluate the treatment effects of melatonin. The results are indicated as the mean ± SEM of three independent experiments. Two-way ANOVA and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, compared with untreated infected cells (0 mM) at each time point. # P < 0.05, compared with the same melatonin concentration at 1 dpi.
In the SK-N-SH cells (Figure 2D and 2E), melatonin at 0.3-1.2 mM showed a strong (~80-100%) reduction effect as observed by the complete inhibition of foci formation in ZIKV-infected cells at 1 dpi. The reduction in the virus yield dropped to ~50-70% for 0.6 and 1.2 mM melatonin at 2 and 3 dpi. The titer of ZIKV decreased to ~35-45% in the presence of 0.15 mM melatonin only at 1 dpi and 0.3 mM melatonin at 2 and 3 dpi. A significant inhibitory effect of melatonin at 0.6 and 1.2 mM was observed at all time points (P < 0.05). The reduction in the virus yield by melatonin treatment in the SK-N-SH cells gradually decreased over time for 0.3 mM (P < 0.05). A significant decrease in the inhibitory effect of 0.6 and 1.2 mM melatonin was found at 3 dpi compared to the effect at 1 dpi (P < 0.05). The results demonstrated that the IC50 of melatonin in SK-N-SH cells was determined to be 0.1844, 0.2906 and 0.3081 mM for days 1, 2 and 3 of ZIKV infections, respectively.
These results revealed that melatonin exhibited antiviral activity against ZIKV production in both cell types and that prophylactic treatment with melatonin exhibited a time- and dose-dependent decrease in the amount of infectious progeny released.
3.3. Ligand-receptor interactions of MEL with ZIKV proteins.
To gain deeper mechanistic insight, we screened for possible intermolecular interactions of melatonin against ZIKV proteins that are vital for successful virus replication via the docking of melatonin to NS1, NS3 helicase, NS2B-NS3 protease, NS5 methyltransferase (NS5 MTase) domain and NS5 RNA-dependent RNA polymerase (NS5 RdRp) domain proteins. The binding affinity energy scores of melatonin against ZIKV proteins are shown in Table1. Our results showed that melatonin exhibits the strongest binding affinity against the NS3 helicase at the ATP/Mn2+ binding site (-7.4 kcal/mol) whereas NS5 at the active site of RdRP (-6.1 kcal/mol) showed the weakest interactions with melatonin. As illustrated in Figure 3, an oxygen atom of the N-acetylaminoethyl side chain in melatonin forms hydrogen bonds with Arg202 and Thr201 by acting as a hydrogen acceptor of the backbone amide hydrogens from both amino acid residues. In addition, an oxygen atom of the methoxy group of melatonin can interact with the hydrogen atoms the guanidinium side chains of Arg459 and Arg462. These extensive hydrogen bonding interactions between melatonin and the amino acid residues of the NS3 helicase give rise to the highest affinity among the other targets (i.e., NS1, NS2B-NS3 protease, NS5 MTase and NS5 RdRp) upon the molecular docking (Table 1). Interestingly, the binding affinity of melatonin to the NS3 helicase is almost as strong as that to QR2, a target of melatonin (29, 30, 32).
Table 1. Parameters for Zika viral proteins and melatonin docking.
No. | Protein | PDB | Function | Position | Grid dimension | Grid center | Affinity (kcal/mol) | ||||
X | Y | Z | X | Y | Z | ||||||
1 | QR2 | 2QWX | Quinone reductase 2 | Melatonin binding site | 45 | 45 | 45 | 23.311 | 16.390 | 15.011 | -7.9 |
2 | NS1 | 5K6K | Active site (Predicted) | 126 | 96 | 100 | -22.038 | -33.679 | -29.624 | -6.3 | |
3 | NS3 | 5JMT | Helicase | ATP/Mn2+ binding site | 46 | 58 | 68 | -18.235 | 19.822 | -22.511 | -7.4 |
4 | NS2B-NS3 | 5LC0 | Protease | Active site | 52 | 72 | 60 | 79.850 | 55.023 | 152.145 | -7.3 |
5 | NS5 MTase domain | 5KQR | RNA replication | mRNA cap-binding site | 42 | 59 | 48 | 1.4 | -9.369 | 13.291 | -6.4 |
Putative RNA-binding site | 42 | 65 | 54 | 2.731 | 3.377 | 10.979 | -6.5 | ||||
SAM-binding site | 52 | 53 | 62 | 10.884 | 4.035 | 1.355 | -6.3 | ||||
Catalytic site | 28 | 45 | 40 | 13.853 | -0.438 | 10.200 | -6.2 | ||||
6 | NS5 RdRP domain | 5U04 | RNA replication | RNA-dependent RNA polymerase (active site) | 100 | 86 | 126 | 24.875 | 71.789 | 114.763 | -6.1 |
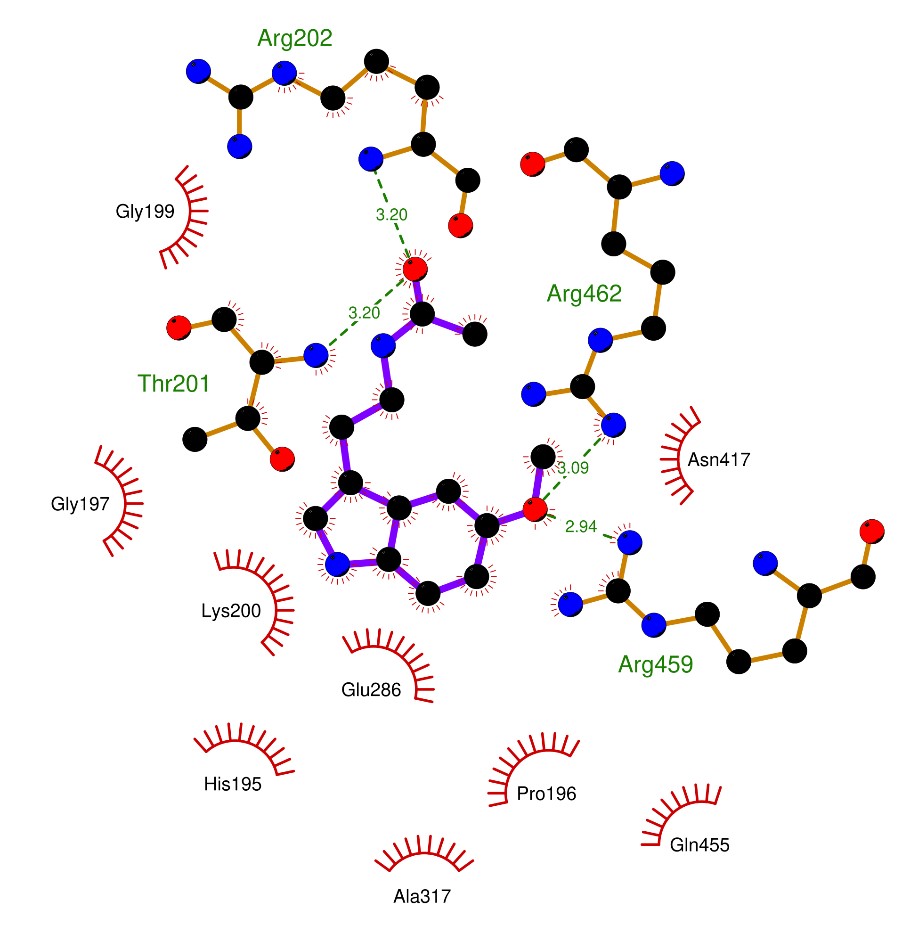
Fig. 3. Potential Ligand-receptor interactions of MEL with ZIKV proteins.
A two-dimensional diagram shows the proposed interaction between melatonin with ZIKV NS3 proteins consisting of intermolecular bonds and close contact amino acid residues generated from LigPlot+ software. H-bonds formed are shown and indicated by green dashed lines.
4. DISCUSSION
Since the outbreak of ZIKV in several countries, many efforts have been made to search for effective antiviral drugs for ZIKV infection treatment. Several FDA-approved drugs such as sofosbuvir (33), bromocriptine (34), ribavirin (35) and other bioactive agents including curcumin (36), epigallocatechin gallate (EGCG) (37) and bicalein (38), have demonstrated possible antiviral actions to reduce the consequences of ZIKV infections. Here, we report for the first time that melatonin, an indoleamine synthesized from tryptophan, has anti-ZIKV activity as evidenced by the decrease in viral yield in cultured Vero and SK-N-SH by treatment with melatonin prior to infection and subsequently during incubation with melatonin after exposure to ZIKV.
In this study, the tested concentrations of melatonin were chosen based on the cytotoxicity assessment and also based on the previous in vitro studies of antiviral effect of melatonin against several other viruses; the effective melatonin concentrations ranged between 0.1-1.2 mM in different cell lines (23, 25, 39-42). Our results demonstrated that the pre- and posttreatment effect of melatonin on ZIKV infection was time- and dose-dependent. The present results showed that at a high, but a non-cytotoxic concentration, melatonin treatment significantly reduced new viral progeny production by more than 50% up to 3 days after being infected with ZIKV; the lower concentrations of melatonin initially caused a gradual decrease in anti-ZIKV activity after 1 dpi but it was ineffective on day 3 of infection. These results identified the effective concentration and duration of treatment with melatonin for ZIKV infection. In addition, the present analysis of the predicted interaction of melatonin with ZIKV proteins revealed the possible underlying inhibitory mechanisms by which melatonin negatively impacts viral replication.
Molecular docking experiments indicated that melatonin binds to the ZIKV NS3 protein more strongly than to any other protein essential for viral replication, with a binding affinity of -7.4 kcal/mol comparable to the reference binding affinity of QR2 [formally the melatonin membrane receptor 3 (MT3)], a target of melatonin, at -7.9 kcal/mol. From a molecular geometry perspective, not only do the hydrogen bonds between melatonin and arginine residues located in proximity to the ATP/Mn2+ binding site contribute to high affinity binding (38), but also the similar configuration of the N-acetylaminoethyl group of melatonin to pyrophosphate (43), in terms of both geometry and bond length (C – C bond = 154 pm, P – O bond = 152 pm), leads to the excellent fit insertion of the N-acetylaminoethyl substituent into the binding pocket. The functional domains of flaviviral NS3 are composed of the N-terminal protease domain that cleaves the viral and host protein precursor and the C-terminal helicase domain responsible for unwinding the viral genome (44). The NS3 protein of the ZIKV plays a pivotal role in viral replication in cells which possess the necessary enzymatic functions together with cooperation of NS5 which then dominates viral genome amplification (45, 46). Thus, the reduction in viral production can be mechanistically explained by the binding of melatonin to the ATP binding region of the ZIKV NS3 helicase domain, which in turn impairs the unwinding ability of NS3 and disrupts the NS3-NS5 interaction. The predicted interaction of melatonin with ZIKV NS3 was also shown for other ligands including luteolin, ivermectin, suramin, dasatinib, panduratin A, EGCG, 4-methoxyphenyl 4-chloro-3-nitrobenzoate and ZINC53047591, which also showed a high binding affinity for the NS3 protein and good drug likelihood properties (47-50). Clearly, NS3 may be a critical target for drug design studies for the control of ZIKV infections.
As a result of earlier studies, melatonin has been suggested for use as an inhibitor of infections because it lowered viremia and the virus titer in targeted cells and, additionally, it reduced the mortality incidence of animals infected with Venezuelan equine encephalomyelitis virus (VEEV) (51), DENV (42), WNV (52), influenza A virus (23), Semliki Forest virus (SFV) (52), rabbit hemorrhagic disease virus (RHDV) (53, 54) and protected against four different swine coronaviruses (25). Moreover, melatonin exhibits several actions against viral infections that would help to control such infections; these actions include antioxidant (40, 54-57), anti-apoptosis (40, 54, 57), and anti-inflammation (23, 27, 53, 57, 58), all of which protect against or prevent viral invasion and the associated molecular and cellular damage. Due to high protective effect and safety of melatonin, the use of this agent as a treatment for patients with COVID-19 is being tested in clinical trials (59-61).
In the present study, we demonstrated the antiviral properties of melatonin against ZIKV in Vero cells derived from the kidney of an African green monkey (Cercopithecus aethiops), which are widely used to study viral infections and in SK-N-SH, a human neuroblastoma cell line, one of the permissive targets of ZIKV infection. Clinically, the central nervous system and neural stem cells are highly permissive to ZIKV infection and exhibit a strong correlation with one of the mechanisms that causes severe neurological disease and microcephaly (62-64). Here, we found that although the prophylactic treatment with melatonin exhibited antiviral activity against ZIKV by reducing the viral yield in both cell types, the two mammalian cell lines were differentially sensitive to the drug. The reduction in viral yield after melatonin treatment of ZIKV-infected SK-N-SH cells was better than that for infected Vero cells. The lower IC50 of melatonin in SK-N-SH cells than in Vero cells suggested the high inhibitory effect of melatonin against ZIKV infection in neurons. As evidence relating to the present of melatonin receptors and other transporters that facilitate melatonin transportation into the cells including glucose transporter 1 and peptide transporter 1 and 2 which may potentiate the action of melatonin in the neuronal cells (65-67). The findings of other studies have also shown that melatonin has a protective role on both Vero cells (68) and SK-N-SH cells (69, 70) against toxic substance-induced cytotoxicity, probably through its antioxidative capacity (16, 71).
The results of numerous studies have documented the ability of melatonin to reduce free radical-mediated damage to the brain in neurological disease models (72, 73). The prophylactic administration of melatonin has demonstrated neuroprotective effects in several human neurodegenerative diseases (74, 75) as well as therapeutic potential against many pathogens such as parasites, bacteria and viruses (26, 76, 77). Melatonin shows to disrupt the coordination of pathogen life cycle and induces the hosts’ defense mechanisms in order to combat with the infection (78, 79). No severe or fatal adverse effects have been observed with the consumption of melatonin; the dose of 1-10 mg/kg is considered a standard dose for threatening circadian rhythm disruption in patients without any toxicological effects after long-term treatment (80). Much higher doses of melatonin have also demonstrated the general lack of toxicity of this endogenous molecule in pregnant animal models (81). Our results show the beneficial effect of melatonin treatment against ZIKV infection, particularly on neuronal cell types. The present results provide a promising strategy for the prophylactic treatment of pregnant women or individuals visiting ZIKV endemic areas with melatonin to prevent or to reduce the development of neurological diseases and to control ZIKV transmission. Further intensive in vitro and in vivo studies are needed to confirm the antiviral activity of melatonin and to further clarify the actions of this non-toxic molecule against ZIKV infection.
5. CONCLUSION
The present results expand the number of viruses that are sensitive to melatonin and support the general anti-viral activity of this endogenous, no-toxic molecule. The findings reported herein suggest the inhibitory effects of melatonin on ZIKV replication involves its possible interaction with the ATP binding region of the ZIKV NS3 helicase. The wide-spread antiviral activities of melatonin (82) support its use in further experimental studies and clinical trials to identify is efficacy as a prophylactic or preventive treatment for not on ZIKV but other viral infections as well.
ACKNOWLEDGEMENTS
This research project is supported by National Science and Technology Development Agency for PP and was supported by Thailand Research Fund (RSA6280035) and Specific League Funds from Mahidol University for PW. We thank Miss Soraya Sangjan for assistance with data collection.
AUTHORSHIP
The contribution of each author was as follows: PP and PW conceived and designed the study; TC, PW, AS and WD performed the research and analyzed the data; MY and WJ conducted the molecular docking; and PW, PG and PP were responsible for data interpretation and the writing of the manuscript. RJR critically reviewed and re-wrote portions of the manuscript. All authors approved the manuscript before submission.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the performance or the presentation of the work reported in this paper.
REFERENCES
Russo FB, Jungmann P, Beltrao-Braga PCB (2017) Zika infection and the development of neurological defects. Cell Microbiol. 19 (6): e12744.
Hancock WT, Marfel M, Bel M (2014) Zika virus, French Polynesia, South Pacific, 2013. Emerg. Infec.t Dis. 20 (11): 1960.
Li H, Saucedo-Cuevas L, Shresta S, Gleeson JG (2016) The neurobiology of Zika virus. Neuron 92 (5): 949-958.
Carod-Artal FJ (2018) Neurological complications of Zika virus infection. Expert Rev. Anti. Infect Ther. 16 (5): 399-410.
Oehler E, et al. (2014) Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro. Surveill. 19 (9): 20720.
Weaver SC, et al. (2016) Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 130: 69-80.
Hamel R, et al. (2015) Biology of Zika virus infection in human skin cells. J. Virol. 89 (17): 8880-8896.
Saiz JC, et al. (2016) Zika virus: the latest newcomer. Front. Microbiol. 7: 496.
Paz-Bailey G, et al. (2018) Persistence of Zika virus in body fluids - Final report. N. Engl. J. Med. 379 (13): 1234-1243.
Dupont-Rouzeyrol M, Biron A, O'Connor O, Huguon E, Descloux E (2016) Infectious Zika viral particles in breastmilk. Lancet 387 (10023): 1051.
Reiter RJ, Tan DX, Kim SJ, Cruz MH (2014) Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 219 (6): 1873-1887.
Bitar RD, Torres-Gomez J, Reiter RJ, Phillips WT (2021) Neural glymphatic system: Clinical implications and potential importance of melatonin. Melatonin Res. 4 (4): 551-565.
von Gall C (2022) The effects of light and the circadian system on rhythmic brain function. Int. J. Mol. Sci. 23 (5): 2778.
Acuna-Castroviejo D, et al. (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71 (16): 2997-3025.
Claustrat B, Leston J (2015) Melatonin: Physiological effects in humans. Neurochirurgie 61.(2-3): 77-84.
Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 65 (1): e12514.
Rommasi F, Nasiri MJ, Mirsaeidi M (2022) Immunomodulatory agents for COVID-19 treatment: possible mechanism of action and immunopathology features. Mol. Cell. Biochem. 477 (3): 711-726.
El-Sokkary GH, Omar HM, Hassanein AF, Cuzzocrea S, Reiter RJ (2002) Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Radic. Biol. Med. 32 (4): 319-332.
Tekbas OF, Ogur R, Korkmaz A, Kilic A, Reiter RJ (2008) Melatonin as an antibiotic: new insights into the actions of this ubiquitous molecule. J. Pineal Res. 44 (2): 222-226.
Vielma JR, et al. (2014) Effects of melatonin on oxidative stress, and resistance to bacterial, parasitic, and viral infections: a review. Acta Trop. 137: 31-38.
Srinivasan V, Mohamed M, Kato H (2012) Melatonin in bacterial and viral infections with focus on sepsis: A review. Rec. Pat. Endoc.r Metab. Immune Drug Discov. 6 (1): 30-39.
Bonilla E, Valero N, Chacin-Bonilla L, Medina-Leendertz S (2004) Melatonin and viral infections. J. Pineal Res. 36 (2): 73-79.
Huang S-H, et al. (2019) Melatonin possesses an anti-influenza potential through its immune modulatory effect. J. Funct. Foods 58: 189-198.
Wongchitrat P, Shukla M, Sharma R, Govitrapong P, Reiter RJ (2021) Role of melatonin on virus-induced neuropathogenesis-A concomitant therapeutic strategy to understand SARS-CoV-2 infection. Antioxidants (Basel) 10 (1): 47.
Zhai X, et al. (2021) Melatonin and other indoles show antiviral activities against swine coronaviruses in vitro at pharmacological concentrations. J. Pineal Res. 71 (2): e 12754.
Silvestri M, Rossi GA (2013) Melatonin: Its possible role in the management of viral infections--a brief review. Ital. J. Pediatr. 39: 61.
Reiter RJ, et al. (2022) Melatonin: highlighting its use as a potential treatment for SARS-CoV-2 infection. Cell. Mol. Life Sci. 79 (3): 143.
Trott O & Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31 (2): 455-461.
Boutin JA (2016) Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin. Ther. Targets 20 (3): 303-317.
Liu L, Labani N, Cecon E, Jockers R (2019) Melatonin target proteins: Too many or not enough? Front. Endocrinol.) 10: 791.
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 8 (2): 127-134.
Calamini B, Santarsiero BD, Boutin JA, Mesecar AD (2008) Kinetic, thermodynamic and X-ray structural insights into the interaction of melatonin and analogues with quinone reductase 2. Biochem. J. 413 (1): 81-91.
Ferreira AC, et al. (2017) Sofosbuvir protects Zika virus-infected mice from mortality, preventing short- and long-term sequelae. Sci. Rep. 7 (1): 9409.
Chan JF, et al. (2017) Novel antiviral activity and mechanism of bromocriptine as a Zika virus NS2B-NS3 protease inhibitor. Antiviral Res. 141: 29-37.
Vicenti I, et al. (2018) Comparative analysis of different cell systems for Zika virus (ZIKV) propagation and evaluation of anti-ZIKV compounds in vitro. Virus Res. 244: 64-70.
Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M (2017) Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res. 142: 148-157.
Sharma N, Murali A, Singh SK, Giri R (2017) Epigallocatechin gallate, an active green tea compound inhibits the Zika virus entry into host cells via binding the envelope protein. Int. J. Biol. Macromol. 104 (Pt A): 1046-1054.
Oo A, Teoh BT, Sam SS, Bakar SA, Zandi K (2019) Baicalein and baicalin as Zika virus inhibitors. Arch. Virol. 164 (2): 585-593.
Valero N, Espina LM, Mosquera J (2006) Melatonin decreases nitric oxide production, inducible nitric oxide synthase expression and lipid peroxidation induced by Venezuelan encephalitis equine virus in neuroblastoma cell cultures. Neurochem. Res. 31 (7): 925-932.
Montiel M, et al. (2015) Melatonin decreases brain apoptosis, oxidative stress, and CD200 expression and increased survival rate in mice infected by Venezuelan equine encephalitis virus. Antivir. Chem. Chemother. 24 (3-4): 99-108.
Rabbani MAG, Barik S (2017) 5-Hydroxytryptophan, a major product of tryptophan degradation, is essential for optimal replication of human parainfluenza virus. Virology 503: 46-51.
Morchang A, et al. (2021) Melatonin inhibits dengue virus infection via the sirtuin 1-mediated interferon pathway. Viruses 13 (4): 659.
Jain R, Coloma J, Garcia-Sastre A, Aggarwal AK (2016) Structure of the NS3 helicase from Zika virus. Nat. Struct. Mol. Biol. 23 (8): 752-754.
Tay MYF, Vasudevan SG (2018) The transactions of NS3 and NS5 in flaviviral RNA replication. Adv. Exp. Med. Biol. 1062: 147-163.
Pathak N, et al. (2020) Zika virus NS3 protease pharmacophore anchor model and drug discovery. Sci. Rep. 10 (1): 8929.
Xu S, et al. (2019) Zika virus NS3 is a canonical RNA helicase stimulated by NS5 RNA polymerase. Nucleic Acids Res. 47 (16): 8693-8707.
Faizan MI, et al. (2019) Structure based identification of potential inhibitors of NS3 protein of Zika virus. Lett. Drug Des. Discov. 16 (7): 761-774.
Kumar D, Sharma N, Aarthy M, Singh SK, Giri R (2020) Mechanistic insights into Zika virus NS3 helicase inhibition by epigallocatechin-3-gallate. ACS Omega 5 (19): 11217-11226.
Sahoo M, Jena L, Daf S, Kumar S (2016) Virtual screening for potential inhibitors of NS3 protein of Zika virus. Genomics Inform. 14 (3): 104-111.
Tan CW, Sam IC, Chong WL, Lee VS, Chan YF (2017) Polysulfonate suramin inhibits Zika virus infection. Antiviral Res. 143: 186-194.
Valero N, et al. (2015) Melatonin, minocycline and ascorbic acid reduce oxidative stress and viral titers and increase survival rate in experimental Venezuelan equine encephalitis. Brain Res. 1622: 368-376.
Ben-Nathan D, Maestroni GJ, Lustig S, Conti A (1995) Protective effects of melatonin in mice infected with encephalitis viruses. Arch. Virol. 140 (2): 223-230.
Crespo I, et al. (2016) Melatonin inhibits the sphingosine kinase 1/sphingosine-1-phosphate signaling pathway in rabbits with fulminant hepatitis of viral origin. J. Pineal Res. 61 (2): 168-176.
San-Miguel B, et al. (2014) Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J. Pineal Res. 56 (3): 313-321.
Valero N, et al. (2005) In vitro, melatonin treatment decreases nitric oxide levels in murine splenocytes cultured with the venezuelan equine encephalomyelitis virus. Neurochem. Res. 30 (11): 1439-1442.
Huang SH, Cao XJ, Liu W, Shi XY, Wei W (2010) Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. 48 (2): 109-116.
Sang Y, et al. (2018) Melatonin ameliorates coxsackievirus B3-induced myocarditis by regulating apoptosis and autophagy. Front. Pharmacol. 9: 1384.
Bonilla E, et al. (2003) Melatonin increases interleukin-1beta and decreases tumor necrosis factor alpha in the brain of mice infected with the Venezuelan equine encephalomyelitis virus. Neurochem. Res. 28 (5): 681-686.
Acuna-Castroviejo D, et al. (2020) Clinical trial to test the efficacy of melatonin in COVID-19. J Pineal Res. 69 (3): e12683.
Rodriguez-Rubio M, et al. (2020) A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): A structured summary of a study protocol for a randomized controlled trial. Trials 21 (1): 699.
Brusco LI, Cruz P, Cangas AV, Gonzalez-Rojas C, Vigo DE, Cardinali DP (2021) Efficacy of melatonin in non-intensive care unit patients with COVID-19 pneumonia and sleep dysregulation. Melatonin Res. 4: 173-188.
Garcez PP, et al. (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352 (6287): 816-818.
Tang H, et al. (2016) Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18 (5): 587-590.
Russo FB, Beltrao-Braga PCB (2017) The impact of Zika virus in the brain. Biochem. Biophys. Res. Commun. 492 (4): 603-607.
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell Endocrinol. 351 (2): 152-166.
Hevia D, et al. (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234-250.
Huo X, et al. (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 62 (4): e12390.
Moravcik R, Okuliarova M, Kovacova E, Zeman M (2014) Diquat-induced cytotoxicity on Vero and HeLa cell lines: Effect of melatonin and dihydromelatonin. Interdiscip. Toxicol. 7 (4): 184-188.
Klongpanichapak S, Phansuwan-Pujito P, Ebadi M, Govitrapong P (2007) Melatonin protects SK-N-SH neuroblastoma cells from amphetamine-induced neurotoxicity. J. Pineal Res. 43 (1): 65-73.
Chetsawang J, Govitrapong P, Chetsawang B (2007) Melatonin inhibits MPP+-induced caspase-mediated death pathway and DNA fragmentation factor-45 cleavage in SK-N-SH cultured cells. J. Pineal Res. 43 (2): 115-120.
Ghosh P, Dey T, Chattopadhyay A, Bandyophyay D (2021) An insight into the ameliorative effects of melatonin against chromium induced oxidative stress and DNA damage: a review. Melatonin Res. 4 (3): 377-407.
Singh M, Jadhav HR (2014) Melatonin: functions and ligands. Drug Discov. Today 19 (9): 1410-1418.
Tuli HS, Kashyap D, Sharma AK, Sandhu SS (2015) Molecular aspects of melatonin (MLT)-mediated therapeutic effects. Life Sci. 135: 147-157.
Meng X, et al. (2017) Dietary sources and bioactivities of melatonin. Nutrients 9 (4): 367.
Loh D, Reiter RJ (2021) Melatonin: regulation of biomolecular condensates in neurodegenerative disorders. Antioxidants 10 (9): 1483.
Elmahallawy EK, et al. (2015) Potential relevance of melatonin against some infectious agents: A review and assessment of recent research. Curr. Med. Chem. 22 (33): 3848-3861.
Tan DX, Reiter RJ (2021) Melatonin reduces the mortality of severely-infected COVID-19 patients. Melatonin Res. 4 (4): 613-616.
He F, et al. (2021) Bacteriostatic Potential of Melatonin: Therapeutic standing and mechanistic insights. Front. Immunol. 12: 683879.
Pereira PHS, Garcia CRS (2021) Melatonin action in Plasmodium infection: Searching for molecules that modulate the asexual cycle as a strategy to impair the parasite cycle. J. Pineal Res. 70 (1): e12700.
Andersen LP, Rosenberg J, Gogenur I (2014) Perioperative melatonin: not ready for prime time. Br. J. Anaesth. 112 (1): 7-8.
Jahnke G, et al. (1999) Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 50 (2): 271-279.
Reiter RJ, Ma Q, Sharma R (2020) Treatment pf Ebola and other infectious diseases: melatonin “goes viral”. Melatonin Res. 3: 43-57.

This work is licensed under a Creative Commons Attribution 4.0 International License