Please cite this paper as:
Pal, P.K., Bhattacharjee, B., Chattopadhyay, A. and Bandyopadhyay, D. 2019. Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Research. 2, 2 (Jun. 2019), 158-184. DOI:https://doi.org/https://doi.org/10.32794/mr11250027.
Review
Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview
Palash Kumar Pala^, Bharati Bhattacharjeea^, Aindrila Chattopadhyayb, Debasish Bandyopadhyaya*
aOxidative Stress and Free Radical Biology Laboratory, Department of Physiology, University of Calcutta, 92, APC Road, Kolkata-700009
bDepartment of Physiology, Vidyasagar College,39, Sankar Ghosh Lane, Kolkata-700006
^Authors have equal contribution
*Correspondence: debasish63@gmail.com; Tel: +91-9433072066
Running title: Melatonin protects against oxidative stress-induced gastrointestinal damage
Received: April 22, 2019; Accepted: June 5, 2019
ABSTRACT
The excessive production of free radicals and/or reactive oxygen species (ROS) in gastrointestinal (GI) tract leads to oxidative damages in GI tissues with development of varied pathological conditions and clinical symptoms. Many endogenous as well as exogenous factors are involved in such pathogenesis, herein, focus was given to the factors of metal toxicity, non-steroidal anti-inflammatory drugs (NSAIDs), ischemia-reperfusion, consumption of high fat diet and alcohol, and different pathological conditions and diseases. Since ROS is more or less involved in the GI damages caused by these factors, therefore attempts have been made to develop appropriate therapeutic agents that possess antioxidant properties. Being a potent antioxidant and free radical scavenger, melatonin was suggested as a potent therapeutic answer to these GI damages. The discovery of different binding sites and receptors of melatonin in the GI tissues further proves its local actions to protect these tissues from oxidative stress. In the review, we attempt to try our best to summarize the current developments regarding the GI injuries caused by oxidative stress and the potential beneficial effects of melatonin on these injuries. The important molecular mechanisms associated with these changes were also highlighted in the discussion. We hope that this review will provide valuable information to consider melatonin as a suitable molecule used for GI tract protection.
Keywords: gastrointestinal tract, oxidative stress, tissue damage, melatonin, antioxidant, protection.
__________________________________________________________________________________________
1. INTRODUCTION
Oxidative stress plays a key role in the pathogenesis of varied clinical conditions caused by ROS (1-3). A growing body of animal studies clearly indicated the harmful effects of oxidative stress on pathogenesis in different organs including heart (4-5), liver (5), lung (6), muscle (7) and GI tract (2-3, 8-9). Among these, GI tract has drawn a great attention from researchers due to its production of large amounts of ROS (1), particularly under ischemia/reperfusion event (10). In addition, harmful substances containing in the ingested food particles and pathogens would cause epithelium-associated intestinal inflammation to enhance the production of inflammatory cytokines and other mediators from the polymorphonuclear neutrophils and macrophages. These eventually further increase intracellular oxidative stress in GI tract (11). Other factors including heavy metal (12-16), non-steroidal anti-inflammatory drugs (NSAIDs) (3, 17), consumption of high fat diet (18) and alcohol (19), ischemia-reperfusion (20) and variety of diseases (11) also cause gastrointestinal injuries. Many more other harmful factors can be mentioned; however, the current review will focus on what we have mentioned above, which are more or less associated with GI tract oxidative stress.
Discovery of the free radical scavenging and antioxidant properties (21) of an essential amino acid tryptophan’s derivative, melatonin, opens up a new avenue in the field of oxidative stress protection. Thereafter, identification of melatonin binding sites or its receptors in the GI tract has promoted the idea of melatonin’s local actions in the GI tract (2, 22-25). Animal studies have provided further evidences to suggest that melatonin, by acting as a potent free radical scavenger (26) and/or by stimulation of different antioxidant enzymes (27), suppresses intra-cellular oxidative stress during inflammation (28-29). Inflammation per se also induces melatonin synthesis in GI tract and the synthetic activity of melatonin directly depends on the degree of inflammation in the GI tissues (24). Considering its location and functions, we hypothesize that melatonin may play a critical role in GI tract, particularly in protection of GI tract from the oxidative stress. These will be discussed below.
2. MODES OF OXIDATIVE STRESS INDUCED TISSUE DAMAGE IN THE GI TRACT
2.1. Endogenous stressors.
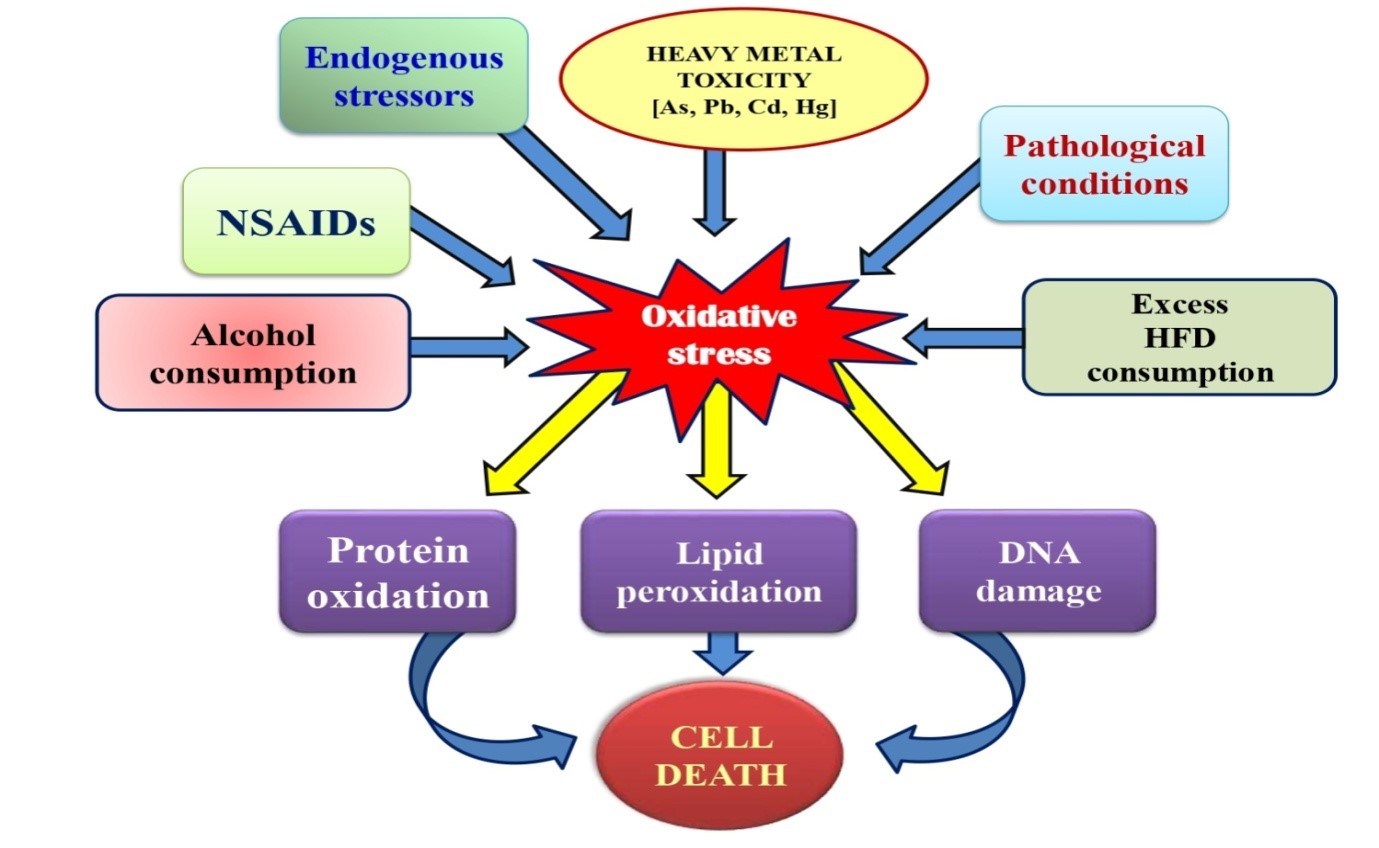
Despite of the fact that numerous endogenous stressors are involved in the excessive production of ROS (30), most studies are focused on the exogenously occurring oxidative stressors in the GI tract as illustrated in Fig. 1. Catecholamines, especially adrenaline, are endogenously occurring substances and they are released from sympathetic nervous system and adrenal medulla into the circulation to balance the normal physiological functions (31). However, due to their potent pro-oxidant and auto-oxidation properties, the uncontrolled and excessive release of adrenaline will generate enormous ROS which will cause harmful effects on the cell/tissue/organ (32-35). Adrenaline has been reported to cause mucosal erosion in mammalian GI tract (36). A recent in vitro study has confirmed the adverse effects of adrenaline in different GI tissues of rats (2). Briefly, adrenaline was found to decrease intracellular levels of necrosis factor-kappa beta (NF-kβ), but increased the levels of lipid peroxidation, protein carbonyl content, nitrate and inflammatory cytokines [tumour necrosis factor-α (TNF-α), interleukin-1 beta (IL-1β) and interleukin-6 (IL6)]. Moreover, activities of different antioxidant enzymes were also enhanced following adrenaline treatment. The results demonstrated the capability of adrenaline in inducing oxidative stress by altering the antioxidative and inflammatory responses in GI tract (2).
2.2. Exogenous stressors.
2.2.1. Heavy metal toxicity.
To maintain a variety of biological functions, some heavy metals are essential at certain concentrations in organisms. However, they become toxic when their concentrations exceed threshold levels. Heavy metal toxicities were associated with various pathologies in animals (37). Not only heavy metals per se but their mixtures with other compounds possess toxicities in organisms (38). Earth crust enriches heavy metals but the pollution is mainly from industrial polluted ground water, mining, sewage sludge, commercial products, urban runoff, contaminated food chain and many others (39). One of the important mechanisms related to heavy metal toxicity is oxidative stress. In depth, these heavy metals, arsenic, lead, cadmium and mercury, hold the ability to generate a wide variety of ROS including superoxide anion (O2•-), nitric oxide (•NO), hydroxyl radical (•OH). These ROS actively participate in metal toxicity and result in oxidative injury in lipids, proteins and DNA (40) which leads to gastrointestinal as well as neuro-, hepato-, nephro- and cardio-toxicities in humans and other vertebrates (5, 41-43). To better understand their toxicities, each of them will be discussed respectively.

Figure 1: Schematic representation of the roles of different endogenous and exogenous stressors on gastrointestinal tissue injuries.
2.2.1.1. Arsenic (As).
As (also termed as protoplastic poison) is widely scattered in the environment that forms diverse chemical complexes in the body with its most common oxidation states of 5+, 3+ and 3-. In nature, As exists as the organic, inorganic and arsine gas, of which inorganic and arsine gas are the most toxic chemical forms. Ingestion, inhalation and absorption through skin are the possible route of As exposure. The contaminated drinking water is the most common source. The estimated lethal dose of inorganic As is 0.6 mg/kg. In mammals, 80-90% absorption of As takes place in the GI tract and then, releases into the blood, where it binds with globin and thereafter is transported throughout the body (43-44). The fact that As causes excessive production of ROS and oxidative tissue injury has been well documented in humans and, its adverse effects on important organs of any individual exposed are devastating (45). As interacts with sulfhydryl group containing proteins and, therefore, jeopardizes functions of several enzymes which are associated with cellular respiration, in some cases, it even disturbs mitosis (45). Arsenic increases O2•-, H2O2 and •OH to induce lipid peroxidation (LPO), and initiates apoptosis. The oxidative stress caused by As alters the activities of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and also non-enzymatic antioxidant i.e., reduced glutathione (GSH) possibly through the formation of monomethyl arsenous (MMA) compounds (45). Notably, covalent binding of As with the thiol group of nitric oxide synthases (NOS) produces monomethylarginine (MMA) compounds which promote the development of different gastrointestinal symptoms described as arsenicosis. Apart from serious GI irritation, As exposure also causes nausea, vomiting and diarrhoea (14). Clinical signs of acute As poisoning in GI tract include painful swallowing, nausea, thirst, burning lips, several abdominal colic, etc. Mechanism of As toxicity in GI tract is associated with epithelial oxidative damage (12). Flora et al. (41) have confirmed the association between the concentration of As and ROS, its interaction with NOS and modulation of signalling cascade pathway via mostly mitogen activated protein kinases (MAPKs). All these events collectively result in the induction of cellular toxicity and apoptosis.
2.2.1.2. Lead (Pb).
Pb is a ubiquitous toxic metal. The features of its ductility, malleability, dense, and corrosion resistance property have made this heavy metal be widely used. Pb is mostly used in the metallic product, battery production, X-ray device etc., leading to exposure of persons working in these industrial areas (46). According to the reference of World Health Organization (WHO), the tolerance of Pb intake should not exceed 25mg/kg/day, otherwise, it causes oxidative stress and organ dysfunctions in mammals (47). Pb has little physiological functions but causes oxidative stress in different vital organs including GI tract (13, 48). This is probably that Pb can mimic some trace elements such as calcium, iron and zinc as a cofactor of several antioxidant enzymes and various intracellularly enzymatic reactions (49). In addition, Pb inhibits glutathione reductase (GR), thereby disturbing the pathway for the conversion of oxidized glutathione (GSSG) to reduced glutathione (GSH), ultimately declines the level of GSH. It was reported that Pb promoted the production of delta-aminolevulinic acid dehydratase (delta-ALAD) which in turn increased the generation of ROS in organisms (50-51). When Pb was incubated with essential unsaturated fatty acid a significant augmentation of malondialdehyde (MDA) was reported (52). Similarly, Pb exposure has also been associated with a high risk of stomach cancer in humans (16). Collectively, Pb exhibits its ability to induce oxidative stress by generation of ROS and reactive nitrogen species (RNS) to damage structures and functions of cell membrane and DNA in different tissues and organs including GI tract in vertebrates (41, 49).
2.2.1.3. Cadmium (Cd).
Cd is a highly pollutant toxicant in the environment and is classified as type-1 human carcinogen by the International Agency for Research on Cancer of USA (53). Cd contamination is widely spread from nickel-Cd batteries, stabilizers, alloys, metal coating and also pigments. Ingestion and inhalation are the main routes of Cd exposure for either occupational or non-occupational persons. The biological life of Cd is around 17-30 years, therefore, it can be accumulated in its primary target organs (liver and kidney) and to some extent in the GI tract (43, 54). Although Cd is a non- Fenton reaction metal, but it generates O2∙-, H2O2 and ∙OH indirectly by the replacement of some metals such as copper (Cu), zinc (Zn) and iron (Fe) which are required for catalytic function of several enzymatic reaction (5). In addition, Cd is reported to induce oxidative stress through other mechanisms including depletion of GSH, binding with thiol-group containing proteins, altering activities of different antioxidant enzymes (SOD, CAT and GPx). It inhibits complex III of the mitochondrial electronic transport chain (ECT), thereby, disrupting mitochondrial membrane structure and triggering the onset of apoptosis (55). Such facts were confirmed by the observation that Cd contamination was found to increase the level of lipid peroxidation resulting in the disruption of proteins, lipids and DNA (56). Although both in vitro and in vivo studies indicated Cd participated in the induction of apoptotic pathways, but the exact mechanisms remain unclear (57).
2.2.1.4. Mercury (Hg).
Hg, a reactive transition metal, exists in a liquid form at room temperature. It however, also can form mercury vapour in the environment to make it be extremely toxic. Intoxication of Hg leads to the development of several disorders including Minamata, Hunter-Russell syndrome and acrodynia (pink disease) (15). Approximately 80% of mercury vapour is absorbed through inhalation and enters into the circulatory system to further distribute to whole body. Since the biological half-life of Hg is 7-10 days, it is cleaned up and metabolized more quickly than other heavy metal from the body (58). The chronic exposure of Hg even at very low concentrations (in the range 0.7–42 µg/m3) will lead to toxicity in a variety of organs, especially GI tract with ulceration and hemorrhage (59). Hg has the great affinity binding to thiol group containing protein like GSH, cysteine and metallothionein (MT) and hence, increases cellular oxidative injury (60). Acute Hg exposure in the form of chloride generally targets GI tract resulting in necrosis of intestinal mucosa, vomiting, bloody diarrhea and abdominal pain possibly due to extensive precipitation of different enterocyte proteins (61).
2.2.2. Non-steroidal anti-inflammatory drugs (NSAIDs).
Use of NSAIDs has been popular for several decades to treat excessive pain, fever and inflammation because of their anti-inflammatory, antipyretic, and analgesia features (3, 17, 62-63). Despite of the fact that NSAIDs such as sulindac, sulindac sulfide, sulindac sulfone, aspirin, indomethacin, acemetacin, tolmetin, etodolac, ketorolac, and oxaprozin exhibit diverse beneficial effects on inflammatory symptoms (64-66), but their uncontrolled use also cause serious complications in different organs, of which, GI tract (17) is the main target. The NSAIDs have varied half-life, high water solubility, detergent (67) and quick absorption properties that eventually increase their bioavailability in the GI tract (68). The high levels of NSAIDs initiate gastric tissue damages and bleeding which further promotes development and progression of inflammation and ulcerative symptoms (11, 69). Several NSAIDs also decrease mucosal microvascular blood flow that is critical in developing gastric ulcers. However, overproduction ROS plays a pivotal role in the development of NSAID associated gastric ulcerations (70-71). NSAIDs, such as aspirin, indomethacin and ibuprofen, inhibit the synthesis of prostaglandins and proliferation of cells, possibly by depleting cellular energy supply that eventually elevates the tissue levels of H2O2 and ·OH in the gastric mucosa leading to oxidative damage (70, 72-74). Indomethacin and piroxicam irreversibly inactivate gastric peroxidise and increase lipid peroxidation rate with concomitant decrease in the levels of antioxidant enzymes, finally leading to excessive production of ROS and gastric ulceration (1, 75). NSAIDs also causes mitochondrial injury by disrupting their transmembrane potential and increasing permeability of transition pore that results in cytochrome c leakage and ROS generation. Consequently, caspase cascade is active to enhance peroxidation of membrane lipids. All these lead to the naive cell towards apoptosis (76). Such sequential responses of varied intracellular components enhance permeability of intestinal mucosa, development of mucosal erosion and the risk of gastric injuries (76).
2.2.3. Ischemia-reperfusion injuries.
Temporary interruption of intestinal blood flow by surgical procedures or, any other patho-physiological processes activate Toll-like receptors (TLRs) that eventually cause serious GI inflammation and tissue injury, a condition termed as ischemia reperfusion (I/R) (20). Actually, re-entry of oxygen to ischemic intestinal tissue results in reperfusion injuries which causes more damage than that of ischemia alone (77) due to massive accumulation of activated neutrophils and ROS (78). High level of oxidized glutathione (79) and the protective role SOD in the I/R tissues suggest a critical role of ROS during I/R induced pathogenesis in the GI tract (10, 80). Similarly, ischemic colitis occurs either due to blood clot induced reduction of blood flow (occlusive), constriction of blood vessels or low systemic blood pressure (non-occlusive) (81). The ischemic colitis also causes lipid peroxidation followed by sepsis and ulceration (82). Results from electron spin resonance spectrometry and low level chemiluminescence identified the occurrence of a sudden burst of oxidation immediately after 2–5 min of reperfusion in ischemic GI tissue (83). The burst of oxidation possibly is derived from multiple sites of mitochondrial ETC (84-85), metabolism of xanthine oxidase (86), endothelial NADPH oxidase (87), prostaglandins (88) and activated neutrophils (89).
2.2.4. High-fat diet.
Regular consumption of high-fat diet (HFD) leads to obesity, cardiovascular disease, dyslipidemia, non-alcoholic steatohepatitis, non-alcoholic fatty liver and insulin resistance along with inflammation and cancer (90-92). It is well documented that innate immune cells stimulated by HFD leads to a transient postprandial inflammation which modulates the intracellular status of inflammation (91-92). Since GI tract is a prime site for HFD induced inflammation, the importance of GI tract on human health has gained a great attention (18). Several studies have pointed out that obesity with HFD may associate with inflammatory changes in GI tract (18). HFD increases the amounts of chylomicrons in the intestine and also changing the composition of gut microbiota which have the direct association with obesity (93). Myeloperoxidase (MPO) is a crucial enzyme responsible for O2 dependent microbial phagocytic activity in the intestine. Intestinal inflammation is associated with elevated level of MPO in the ileum which ultimately induces infiltration of polymorphonuclear neutrophil and macrophage and the overproduction of pro-inflammatory cytokines (TNF-α) (93). de La Serre et al. (93) also demonstrated that increased level of MPO enzyme activity, indirectly increases the activation of TLR4, which further suggests the activation of inflammatory response due to enhancement of enterobacteria. The imbalance between the intracellular levels of antioxidants and oxidants is also potent factor for intestinal inflammation induced by HFD. This imbalance is due to overproduction of inducible nitric oxide synthase (iNOS) which in turn alters permeability of the intestinal membrane (94). Moreover, mitochondria are the primary source of ROS (95), which increases the lipid peroxidation to initiate an intermediate chain reaction to form more O2•- and other ROS (96-97). HFD elevates circulatory free fatty acid (FFA) which then either enters in the mitochondria for oxidation or, is esterified to form triglycerides (TG), thus, increasing the mitochondrial β-oxidation and production of ROS (98). However, the exact pathway responsible for these changes is unclear, and further studies are required to address HFD induced oxidative stress in the GI tract.
2.2.5. Alcohol consumption.
Alcohol can be generated in vivo from the pyruvate to acetaldehyde by alcohol dehydrogenase (98). In GI tract, a significant amount of ethanol is from diet and mainly metabolized in the liver. On other hand, ethanol can also be oxidized into carbon dioxide (CO2) and water (H2O) in the GI tract (99). Consumption of alcohol reduces the metabolism of certain bile-acids and may affect the intestinal microbiota (100). Acute and chronic consumption of alcohol induces oxidative stress by the generation of ROS and disturbs cellular antioxidant levels. Ethanol intoxication increases the production of carbon-centred radicals and H2O2, leading to the dysfunction of endothelium, ischemia of gastric mucosa and microcirculatory disorders (19). Consequently, ROS generated by alcohol modulates or damages the structural proteins, lipid molecules and DNA that in turn inactivates numerous enzyme transporters, transcriptional machinery, etc. Excessive production of ROS ultimately increased the level of lipid peroxidation, an indication of tissue damage, possibly by activation of different enzymes, viz., alcohol dehydrogenase, cytochrome P4502E1 (CYP2E1), aldehyde dehydrogenase and catalase (101). Additionally, oxidative stress induced by alcohol suppresses the activity of first line defense antioxidant enzymes, such as Cu-Zn SOD and Mn-SOD in cytosol and mitochondrial matrix, respectively (102).
2.3. Pathological conditions and diseases.
GI tract serves as one of the major sites for ROS generation to possess epithelial lining as a protective barrier for pathogens. GI tract directly contacts with the ingested food and pathogens which are causative factors of inflammation by activation of epithelium, polymorphonuclear neutrophils and macrophages. These immune cells generate inflammatory cytokines and other cellular mediators causing overproduction of intracellular ROS. The oxidative stress leads to development of numerous pathophysiological conditions including gastric ulcers, diverse malignancies and diseases (11). Some disorders which amplify the level of ROS in the GI tract promoted the vicious cycle to increase the severity of these diseases (11).
Gastroesophageal reflux disease (GERD) associated factors (acid, bile and inflammation) are found to increase the level of ROS either by decreasing endogenous antioxidants or, enhancing the expression of ROS-inducible genes or, both (103). In case of Barrett’s esophagus and esophageal adenocarcinoma, unconjugated bile acids are known to act as potent cyclooxygenase-2 (COX-2) inducer which in turn induces ROS generation and activates the PA3K/AKT and ERK1/2 pathways (104). Similarly, levels of lipid peroxidation and 8-hydroxy-deoxyguanosine (as oxidative stress markers) are reported to be elevated in tissues of esophageal squamous cell cancer (105). Moreover, the reduced levels of SOD are evidenced to increase ROS accumulation in most of the gastroduodenal inflammatory diseases (106-107). On the other hand, phagocytic leukocytes seem to be the prime source of ROS generations in chronic inflammatory diseases, such as Helicobacter pylori induced gastritis and Irritable Bowel disease (IBD). Inflammation induced infiltration of neutrophils and macrophages in the gastric mucosa leads to overproduction of ROS, thus worsening the symptoms in the GI tract (11).
In majority of the peptic ulcer disease and gastritis, H. pylori is known to play key role by inducing non-phagocytic NADPH oxidase and altering proinflammatory cytokine production, thus enhancing the production of ROS in the gastric epithelial pit cells (108-111). The elevated mucosal level of ROS was observed in patients with duodenal ulcer and severe duodenitis (112). On the other hand, enhanced lipid peroxidation along with decreased GSH levels clearly suggested that depletion of endogenous antioxidants may also lead to gastric ulcers (113-114). Prolonged exposure to ROS triggers genome damage and leads to uncontrolled activation of proto-oncogenes, mutations in oncogene/tumor suppressor gene and chromosomal aberrations (11). In case of IBD, inflammation induced phagocyte accumulation results in generation of O2·-, H2O2, and HO·, thus increases the risk of further tissue damage by enhancing membrane lipid peroxidation (115-116). Noteworthy, enteric commensal bacteria associated with the epithelia produce massive ROS that eventually alters/disrupts different intracellular proteins responsible for regulating diverse signalling pathways and affects various physiological functions of the host cell (117). Thus, involvement of ROS in the diverse gastrointestinal diseases is well documented; however, the exact underlying mechanisms are not clearly understood yet and require further investigations.
3. PREVENTIVE STRATEGIES
In order to minimize or, protect the tissue damage of GI tract from varied stressors, numerous approaches have been adopted so far, but all of these attempts remain to be improved for their efficacy. Some of the approaches are discussed herein.
3.1. Chelation therapy against heavy metal toxicity.
The principle of chelation therapy is to remove heavy metals from the body by different chelating agents. Some of them are discussed herein. The 2,3-dimercaptopropanol (BAL; also known as British Anti-Lewisite) is clinically used to treat patients with diverse metal toxicities (118). BAL chelates various toxic metals including inorganic mercury, antimony, bismuth, cadmium, chromium, cobalt, gold, and nickel, to form the stable products in vivo (118). However, profound side effects of BAL have restricted its use in mammals (119-120). 2,3-Dimercapto-1-propanesulfonic acid (DMPS) and its sodium salt (unithiol) are reported to be potentially chelating agents of different heavy metals. In children, DSMPS was found to effectively reduce the circulating level of lead (121) possibly through the organic anion transport system (122). The similar chelating properties were also reported for meso-2,3-dimercaptosuccinic acid (DMSA), calcium disodium ethylenediaminetetraacetic acid (CaNa2EDTA) (123). In addition, 𝛼-lipoic acid quenches ROS under both in vitro and in vivo systems (124-125); it also chelates a variety of heavy metals (such as iron, copper, mercury, and cadmium), thus protecting different cells/tissues damage from oxidative stress (124, 126). However, none of these approaches has been tested in the gastrointestinal tissues under heavy metal toxicity.
3.2. Use of antioxidants against heavy metal toxicity.
The popular antioxidant vitamin E was used for this purpose (128). It reduces several transition metals, for example cupric ions (Cu2+) to cuprous (Cu1+) and ferric ions (Fe3+) to ferrous (Fe2+) and vitamin E was found to be effective against silver (118) and lead (128) induced cell/tissue damage. In Cd intoxicated tissues, vitamin E not only reduced the accumulation of Cd and rate of lipid peroxidation but also restored the levels of different endogenous antioxidants, clearly indicating its antioxidant properties against Cd toxicity (129). N-acetylcysteine or, N-acetyl-L-cysteine (NAC), another antioxidant, protected against toxicities from different heavy metals in organs of mammals (130-132). Interestingly, several plant extracts were also found to be effective in protecting tissues from oxidative stress induced by heavy metals. For example, aqueous extracts of bark of Terminalia arjuna (5), curry leaf (Murraya koenigii) (133) and tulsi leaf (Ocimum sanctum) (134) were reported to act as potent antioxidants in protecting diverse mammalian tissues from Cd toxicity. Their effects in GI tract remain to be tested.
3.3. Development of alternatives for NSAIDs.
As to NSAIDs, several novel alternatives have been developed with expected no or least side effects, but ultimately these new products have still failed for the expectation to reduce their gastric ulcerogenesis (135). For example, nitric oxide (NO) is a potent vasodialator and inhibitor of neutrophil activation, thus, the NO moiety was incorporated into the classic NSAIDs to form NO containing NSAID (NO-NSAIDs) which is supposed to overcome the mucosal injuries (136). The NO-NSAID and its derivatives were unable to reduce the suppression of gastric prostaglandin synthesis and thus, it had limited effect on GI mucosal injuries (137-139). Similarly, the combination of a prostaglandin analogue and NSAID was used, but such attempt was finally neglected due to the severe side effects (140). Numerous other attempts have been made. These include enteric coating technology, parenteral administration, development of pro-drugs, synthesis of basic molecules and co-administration with prostaglandins or, acid secretion inhibitors or, cyclooxygenase-2 (COX-2) inhibitors (141-142), but none of them was recommended due to their unwanted reactions and gastric bleeding properties. Although synthesis and application of any potent antioxidant (such as cysteamine) tagged amide derivatives of different NSAIDs (for example diclofenac acid, tolfenamic acid, ibuprofen and indomethacin) were reported to reduce or, overcome the gastric ulcerogenesis in different mammalian models, further study for human application is required (3).
4. MELATONIN: AS A POSSIBLE THERAPEUTIC ANSWER TO OXIDATIVE STRESS
Melatonin passes through any biological membrane into cell/tissue/organ with ease due to its lipophilic nature and its specific transporters (2, 3, 26-27, 143). This advantage blesses this potent antioxidant to protect diverse cell/tissue from oxidative damage (144-145). Melatonin is highly effective to scavenge the most reactive and harmful •OH (21). Notably, uniqueness of melatonin lies in its pleiotropic actions on cells/tissues that marks its difference from other classic antioxidants. Apart from serving as a direct broad-spectrum antioxidant (21) melatonin also upregulates antioxidant enzymes and downregulates pro-oxidative enzymes to function as an indirect antioxidant (27). Therefore, it is necessary to evaluate the functional potentiality of melatonin in regulation of intra-cellular oxidative status in diverse cell/tissues/organ. Among them, GI tract (1-2, 8-9, 71, 146) has become the favorite site of research (144). This is also the focus of this review.
Antioxidant actions of melatonin on its target cell/organ under oxidative stress environment are conferred through two principle pathways, the receptor-independent and receptor-dependent ones (1, 27, 147). Such conclusion was derived from the observations that melatonin directly interacts with ROS and also upregulates the levels of mRNA and activities of different antioxidant enzymes including SOD, CAT, GPx, GSH, GR, glucose-6-phosphate dehydrogenase (G6PD) and gamma-glutamylcycteine synthase under oxidative stress (27, 144, 147). The details are described following.
4.1. Receptor dependent actions of melatonin.
Confirmation of the paracrine actions of melatonin in the GI tract came from the identification of melatonin specific receptors and/or its binding sites in the intestines of vertebrates (24-25) and the participation of GI derived melatonin in regulation of peripheral circulation of GI tract (24, 71, 148). Furthermore, melatonin was found to interact and activate its membrane receptors in GI tissues. Melatonin acts primarily through the two G-protein coupled membrane receptors- MT1 and MT2; however, another receptor MT3 (human quinone reductase 2) is also known to involve in melatonin signalling (149). Apart from its membrane receptors, melatonin also binds to its nuclear receptors which belong to the RZR/ROR orphan receptor family. Melatonin nuclear receptors have three subtypes- α, β and γ, of which α subtype possesses another four splicing variants (150). Activation of these receptors conveys the signal of melatonin to its target cell, thus exerting its physiological as well as pharmacological effects as a pleiotropic molecule (151). MT1 receptor activation stimulates G proteins; however, the same signal inhibits cAMP signalling pathways (152). In contrary, activation of MT2 receptor regulates phosphoinositide signal trans­duction pathways with the inhibition of the pathways associated with adenylyl cyclase and guanylyl cyclase (152). Under oxidative stress, melatonin acts on these receptors to activate/inhibit these receptors, respectively to precisely regulate the activities as well as expressions of a variety of antioxidant and prooxidant enzymes. The outcomes are to enhance the synthesis of other endogenous antioxidants and to reduce ROS formation. All these render its indirect antioxidant activity (27). For example, melatonin upregulates the activities of SOD, CAT, GPX, glutathione reductase (GR), glucose-6-phosphate dehydrogenase (G-6-PD) and gamma-glutamylcycteine synthase during stressful conditions (27).
4.2. Receptor independent actions of melatonin.
Melatonin can directly scavenge the highly toxic peroxynitrite anion, hydroxyl-, peroxynitrite-, and peroxyyl radicals (95, 143-144, 153). It can also quench singlet oxygen (95, 153) to protect membrane lipid peroxidation (154) under both acute and chronic inflammation (155-156). Such free-radical scavenging activity of melatonin does not require any receptor at the target cells. The results from numerous in vitro and in vivo studies have unequivocally proven the potent direct antioxidant properties of melatonin (155, 157-158). Interestingly, metabolites of melatonin generated from interaction with ROS also possess strong antioxidant properties. These metabolites are not only capable of scavenging highly toxic hydroxyl radical, peroxynitrite anion and peroxy radical, but also quenches singlet oxygen (153, 156, 159). Notably, these melatonin metabolites can cooperate with other antioxidants to maximum their protective effects on integrity of cellular membranes under oxidative stress (154, 160).
4.3. Abundance of melatonin in the gastrointestinal tract.
Melatonin was first reported to be present in the GI tract of rat (161) and mainly localized in the enterochromaffin cells (EC) of the digestive mucosa. These was identified by immunohistological techniques (24, 161) and quantified by radioimmunoassay (RIA) and high performance liquid chromatography (162-164). The auto-radiographic studies depicted its maximum binding in the mucosa and intestinal villi (165). At the sub-cellular level, strongest melatonin binding was found in the nuclear, followed by the microsomal, mitochondrial, and cytosolic fractions (166-167). The arylalkylamine-N-acetyl transferase (AANAT), the rate limiting enzyme in melatonin biosynthesis, was confirmed in the GI tract of numerous vertebrates (2, 8, 168-171). Interestingly, hydroxyindole-O-methyltransferase (HIOMT), the last enzyme in melatonin biosynthesis pathway, was also detected in the digestive mucosa (172). Collectively, all these observations confirmed the presence of endogenous melatonin biosynthesis in the cells of the GI tract.
The biosynthetic process of melatonin in the GI tract is the same as in the pinealocyte with four-steps. First, GI tract cells take up L-tryptophan (Trp) from the circulation and convert it to 5-hydroxy-Trp (5-HTP) by the enzyme Trp-5-monooxygenase or, hydroxylase (173). Thereafter, 5-HTP is decarboxylated to form serotonin (5-hydroxytryptamine, 5-HT) by L-aromatic amino acid decarboxylase (174). Then, AANAT acetylates 5-HT to form N-acetyl serotonin (NAS) (175). Finally, NAS is O-methylated by the enzyme- HIOMT to form melatonin (176).
4.4. Melatonin protects gastrointestinal tissue from oxidative damage.
Melatonin is found to protect individuals from Cd-induced neurotoxicity either by inhibiting the imbalance in mitochondrial fusion and fission (177-178), or by inducing transcription factor EB- mediated autophagy (179). Melatonin also prevents toxicities of Hg (180) and As (181) in different tissues and organs. However, information regarding the protective effects of melatonin on heavy metal toxicities in gastrointestinal tissues is scarce. A study has reported the ameliorative effects of melatonin on Pb-induced oxidative damage in the stomach and duodenal tissues (182) and it showed that exogenous administration of melatonin decreased the levels of lipid peroxidation and protein carbonyl content, restored the activities of different endogenous antioxidants and preserved their histological structures in rats treated with Pb (182).
The protective effects of melatonin in the GI tissue against diverse oxidative stressors promoted the idea that melatonin may also have protective effects on NSAID associated gastric injuries (Fig. 2). The data of melatonin on injuries caused by a variety of stressors in the GI tract (71, 146, 183-184) suggested the potentially beneficial effects of melatonin against NSAID-induced gastric tissue injury. Actually, melatonin exhibited preventive effects on gastric damages caused by indomethacin, aspirin as well as piroxicam (185-186). A concomitant increase in circulating melatonin with aspirin induced acute gastritis indicated an inducible feature of melatonin synthesis in GI tract under oxidative stress, which is supposed to accelerate the ulcer healing process (185). In the case of diclofenac induced intestinal damage, application of melatonin improved the intestinal permeability status and restored mucosal integrity (187-188), possibly through the restoration of membrane potential and energy metabolism in the mitochondria, thus, reducing apoptosis (189).This is supported by melatonin to reduce electron leakage from the ETC and to elevate mitochondrial respiration and synthesis of ATP by promotion of the activities of complex I and IV (95, 147, 190). On other hand, melatonin prevents proteins, lipids and DNA from oxidative damage (144, 191-192). Moreover, melatonin enhanced the activities of gastric peroxidise, SOD and catalase to decrease of •OH (193) and reduces the NSAID induced oxidative damage in GI tract (194).
Among the diverse actions of melatonin in the GI tract, its protective roles against ischemia-reperfusion induced intestinal injuries were also documented. Administration of melatonin prior to ischemia or in the initiation of reperfusion in the intestinal tissues reduced the deleterious effects by over-production of ROS and/or RNS (71, 146, 183, 195-199). In different pathophysiological conditions, administration of melatonin not only suppressed gastric injuries, but also promoted its healing process through MT2 mediated pathway. In brief, MT2 activation enhances the expression of matrix metalloproteinase-2 (MMP-2), while it decreases the levels of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinases-2 (TIMP-2), thus reducing the endogenous level of ROS in the GI tissue (200-201). Several other mechanisms also contribute to the protective effects of melatonin on GI tract. For example, melatonin inhibits secretion of gastric acid and infiltration of neutrophils, promotes mucosal blood flow into ulcer bed, bicarbonate secretion in the duodenum, and prostaglandin synthesis (186, 202-203). These are illustrated in Figure 2.
4.5. Melatonin as a potent anti-inflammatory agent in the GI tract.
Apart from being a potent antioxidant, melatonin is also an important anti-inflammatory agent in different organs including the GI tract (204-211). NF-kβ, a crucial transcription factor highly sensitive to oxidative stress, upregulates expression of several pro-inflammatory genes to induce a variety of cellular responses associated with inflammation (205, 209, 212). Melatonin can modulate NF-kβ signalling pathways to exert its anti-inflammatory role (206, 209, 213-214). Under diverse pathophysiological conditions, melatonin suppresses the intracellular levels of different pro-inflammatory cytokines including IL-1β, IL-2, IL-6, interferon-gamma (IF-γ) and tumor necrosis factor (TNF)-alpha, while it enhances the expression of anti-inflammatory cytokines IL-10 and tumor growth factor-β (207, 209, 215). Thus, melatonin counteracts inflammation associated intrinsic apoptotic pathway (216) in the GI tract. In addition, melatonin protects the endothelial cells from LPO-induced overproduction of NO by inhibiting the expressions of iNOS and cyclooxygenase-2 (COX-2) (217, 219). However, further studies are essential to elucidate the anti-inflammatory role of melatonin against diverse modes of inflammation.
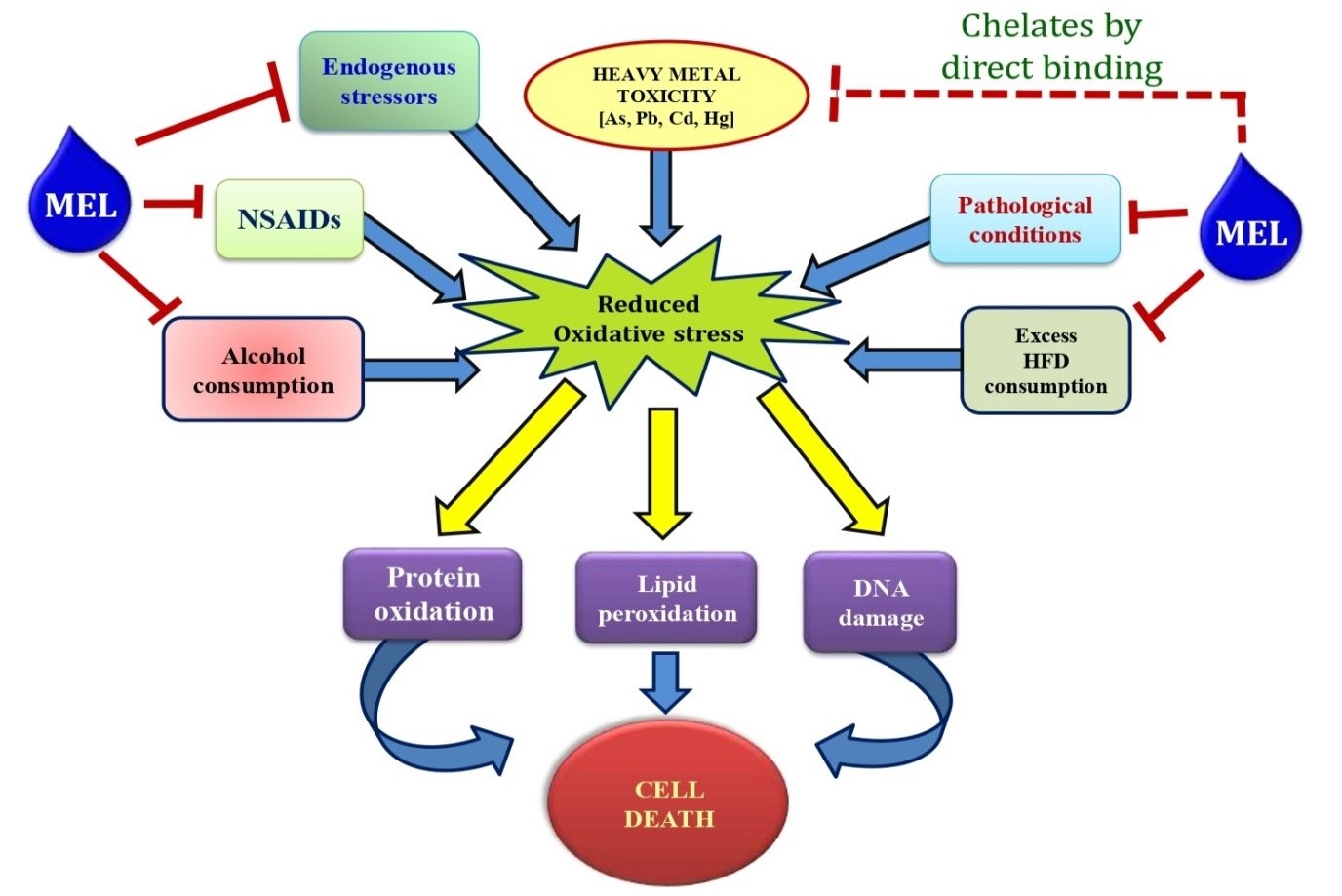
Figure 2: Schematic representation of the protective roles of melatonin on gastrointestinal tissue injuries caused by different endogenous and exogenous stressors.
5. FUTURE PERSPECTIVES
Collectively, these factors discussed above cause GI damage by a common feature that they enhance the production of intracellular ROS. Many attempts have been made to overcome the oxidative stress and GI injuries caused these factors, however, none of these attempts have matched the expectation. It seems that if an antioxidant also exhibited anti-inflammatory activity it would be the suitable candidate for this purpose. Melatonin in this regard is an ideal molecule. It is a potent free radical scavenger with anti-inflammatory property; in addition, it has no, or low cytotoxicity. From this point of view, melatonin would be one of the best molecules to fight the gastrointestinal tissue injury associated with oxidative stress. To understand the protective mechanisms of melatonin in the molecular level requires further well-designed investigations.
ACKNOWLEDGEMENTS
Dr. Palash Kumar Pal gratefully acknowledges the receipt of UGC Dr. D. S. Kothari Post Doctoral Fellowship (BL/16-17/0502), Govt. of India. A financial assistance as Senior Research Fellowship (SRF) under DST-INSPIRE program (IF140691), Govt. of India to Bharati Bhattacharjee is also thankfully acknowledged. Dr. Aindrila Chattopadhyay is supported by funds available to her from Department of Science and Technology, Govt. of West Bengal. Prof. DB is also supported from departmental BI grant of University of Calcutta. DB also gratefully acknowledges the support he received from DST-PURSE Program awarded to University of Calcutta. Prof. DB gratefully acknowledges the contribution of the Editor-In-Chief of Melatonin Research in critically reading and editing of the manuscript which has definitely improved the scientific and readership quality of the article.
AUTHORSHIP
Dr. DB and Dr. AC revised the manuscript critically and approved it. Dr. PKP contributed to conception, prepared figures, drafted the manuscript and edited it. BB contributed in drafting of manuscript.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
Bandyopadhyay D, Biswas K, Bandyopadhyay U, Reiter RJ, Banerjee R (2000) Melatonin protects against stress-induced lesions by scavenging the hydroxyl radical. J. Pineal Res. 29: 143–151.
Pal PK, Bhattacharjee B, Ghosh A, Chattopadhyay A, Bandyopadhyay D (2018) Adrenaline induced disruption of endogenous melatonergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Res. 1: 109–131. doi: 10.32794/mr11250007.
Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyaya D (2019) Melatonin as an armament against non-steroidal anti-inflammatory drug (NSAID) induced gastric injury: An overview. Melatonin Res. 2: 116-138; doi: 10.32794/mr11250015.
Ghosh A, Bose G, Dey T, Pal PK, Mishra S, Ghosh AK, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cardiac damage induced by a combination of high fat diet and isoproteronol exacerbated oxidative stress in male Wistar rat. Melatonin Res. 2: 9-31. DOI: 10.32794/mr11250009.
Bhattacharjee, B, Pal, PK, Ghosh, AK, Mishra A, Chattopadhyay A, Bandyopadhyay D (2019) Aqueous bark extract of Terminalia arjuna protects against cadmium-induced hepatic and cardiac injuries in male Wistar rats through antioxidative mechanisms. Food Chem. Toxicol. 124: 249–264. https://doi.org/10.1016/j.fct.2018.12.008.
Ryrfeldt A, Bannenberg G, Moldeus P (1993) Free radicals and lung disease. Br. Med. Bull. 49: 588–603.
Jackson MJ, O’Farrell S (1993) Free radicals and muscle damage. Br. Med. Bull.49: 630–641.
Pal PK, Hasan NK, Maitra SK (2016a) Gut melatonin response to microbial infection in carp Catla catla. Fish Physiol. Biochem. 42: 579–592. doi: 10.1007/s10695-015-0161-7.
Pal PK, Hasan NK and Maitra SK (2016b) Temporal relationship between the daily profiles of gut melatonin, oxidative status and major digestive enzymes in carp Catla catla. Biol. Rhythm Res. 47: 755–771. doi: 10.1080/09291016.2016.1191697.
Itoh M, Guth PH (1985) Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology 88: 1162–1167.
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94: 329–354. doi:10.1152/physrev.00040.2012.
Tay CH, Seah CS (1975) Arsenic poisoning from anti-asthmatic herbal preparations. Med. J. Aust. 2: 424-428.
Henning SJ, Leeper LL (1984) Duodenal uptake of lead by suckling and weanling rats. Bioi. Neonate 46: 27-35. doi: 10.1159/000242029.
Campbell JP, Alvares JA (1989) U.S. Public Health Service. Toxicological profile for arsenic.USEPA, Washington, DC. Acute arsenic intoxication. Am. Fam. Physician 40: 93-97.
Zalups RK (2000) Molecular interactions with mercury in the kidney. Pharmacol. Rev. 52: 113-143.
Koh DH, Bhatti P, Coble JB, Stewart PA, Lu W, Shu XO, Ji BT, Xue S, Locke SJ, Portengen L, Yang G, Chow WH, Gao YT, Rothman N, Vermeulen R, Friesen MC (2014) Calibrating a population-based job-exposure matrix using inspection measurements to estimate historical occupational exposure to lead for a population-based cohort in Shanghai, China. J. Exp. Sci. Env. Epid. 24: 9–16. doi: 10.1038/jes.2012.86.
Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J (2018) A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 9: 143-150. http://dx.doi.org/10.14336/AD.2017.0306.
Fritsche KL (2015) The science of fatty acids and inflammation. Adv. Nutr. 6: 293S–301S. doi: 10.3945/an.114.006940.
Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide and peroxynitrite: the good, the bad and the ugly. Am. J. Physiol. 271: C1424–C1437. doi: 10.1152/ajpcell.1996. 271.5.c1424.
Victoni T, Coelho FR, Soares AL, de FA, Secher T, Guabiraba R, Erard F, de Oliveira-Filho RM, Vargaftig BB, Lauvaux G, Kama MA, Ryffe B, Moser R, Tavares-de-Lima W (2010) Local and remote tissue injury upon intestinal ischemia and reperfusion depends on the TLR/MyD88signalingpathway. Med. Microbiol. Immunol. 199: 35–42.
Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1:57–60.
Kvetnoy IM, Ingel IE, Kvetnaia TV Malinovskaya NK, Rapoport SI, Raikhlin NT, Trofimov AV, Yuzhakov VV (2002) Gastrointestinal melatonin: cellular identification and biological role. Neuro. Endocrinol. Lett. 23:121–132.
Rakhlin NT, Kvetnoy IM, Tolkachev VN (1975) Melatonin may be synthesized in enterochromafinne cells. Nature 255: 344–345.
Bubenik GA (1999) Localization and physiological significance of gastrointestinal melatonin. In: R Watson (eds.) Melatonin in health promotion. CRC press, Boca Raton, Florida, pp. 21–39.
Dobocovich M, Mankowsha M (2005) Functional MP1 and MP2 melatonin receptors in mammals. Endocrine 27: 101–110. doi: 10.1385/ENDO:27:2:101.
Reiter RJ, Tan DX, Acuna-Castroviejo D, Burkhardt S, Karbownik M (2000) Melatonin: Mechanisms and actions as an antioxidant. Curr. Top. Biophys.24: 171–183.
Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36: 1–9.
Ozdemir D, Uysal N, Tugyan K, Gonenc S, Acikgoz O, Aksu I, Ozkan H (2007) The effect of melatonin on endotoxemia-induced intestinal apoptosis and oxidative stress in infant rats. Intensive Care Med. 33: 511–516.
Trivedi PP, Jena GB (2013) Melatonin reduces ulcerative colitis-associated local and systemic damage in mice: investigation on possible mechanisms. Dig. Dis. Sci. 58: 3460–3474.
Khansari N, Shakiba Y, Mahmoudi M (2009) Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 3: 73–80. doi: 10.2174/187221309787158371.
Gavrilovic L-J, Stojiljkovic V, Kasapovic J, Pejic S, Todorovic A, Pajović B-S, Dronjak S (2012) Chronic physical stress changes gene expression of catecholamine biosynthetic enzymes in the adrenal medulla of adult rats. Acta Vet-Beograd. 62: 151–169. doi: 10.2478/acve-2014-0027.
Liu X, Wu W-K, Yu L, Sung J-J, Srivastava G, Zhang S-T, Cho C-H (2008) Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J. Cell Biochem.105: 53–60. DOI: 10.1002/jcb.21802.
Yao H, Duan Z, Wang M, Awonuga A-O, Rappolee D, Xie Y (2009) Adrenaline induces chemoresistance in HT-29 colon adenocarcinoma cells. Cancer Genet. Cytogenet. 190: 81–87. doi: 10.1016/j.cancergencyto.2008.12.009.
Andrea Č, Lada Ž, Dijana Ž, Ninoslav D, Vladan B, DraganaD, Biljana SP (2014) Protective effect of dry olive leaf extract in adrenaline induced DNA damage evaluated using in vitro comet assay with human peripheral leukocytes. Toxicol. in Vitro 28: 451–456. doi: 10.1016/j.tiv.2013.12.014.
Rudra S, Mukherjee D, Dutta M, Ghosh A-K, Dey M, Basu A, Pattari S-K, Chattopadhyay A, Bandyopadhyay D (2014) Orally administered melatonin protects against adrenaline-induced oxidative stress in rat liver and heart: Involvement of antioxidant mechanism(s). J. Pharm. Res. 8: 303–320. http://jprsolutions.info/files/final-file-56bff9adee9ad8.28733292.pdf.
Raugstad T-S, Svanes K, Ulven A, Molster A (1979) Interaction between acute gastric ulcer and epinephrine-induced mucosal erosions in the rat: the significance of gastric acid secretion. Digestion 19: 70–72. doi: 10.1159/000198325.
Jaishankar M, Mathew BB, Shah MS, Gowda KRS (2014) Biosorption of Few Heavy Metal Ions Using Agricultural Wastes. J. Environ. Pollut. Human Health 2: 1–6. doi: 10.12691/jephh-2-1-1.
Wang G, Fowler BA (2008) Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol. Appl. Pharmacol. 233: 92–99. doi: 10.1016/j.taap.2008.01.017.
Morais S, Costa FG, Pereira ML (2012) Heavy metals and human health. In: Oosthuizen J, editor. Environmental health– emerging issues and practice. 10: 227–246. doi: 10.5772/29869.
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metalions. Free Radic. Biol. Med. 18: 321–336.
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 128: 501–523.
Chen F, Ding M, Castranova V, Shi X (2001) Carcinogenic metals and NF-𝜅B activation. Mol. Cell Biochem. 222: 159–171.
Richardson JB, Dancy BCR, Horton CL, Lee YS, Madejczyk MS, Xu ZZ, Ackermann G, Humphrey G, Palacios G, Knight R, Lewis JA (2018) Exposure to toxic metals triggers unique responses from the rat gut microbiota. Sci. Rep. 8: 6578. doi:10.1038/s41598-018-24931-w.
Evans CD, LaDow K, Schumann BL, Savage RE Jr, Caruso J, Vonderheide A, Succop P, Talaska G (2004) Effect of arsenic on benzo[a] pyrene DNA adduct levels in mouse skin and lung. Carcinogenesis 25: 493-497. doi: 10.1093/carcin/bgg199
Shi H, Shi X, Liu KJ (2004) Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell Biochem. 255: 67-78.
Nava-Ruíz C, Méndez-Armenta M (2013) Cadmium, lead, thallium: occurrence, neurotoxicity and histopathological changes of the nervous system in Pollutant Diseases, Remediation and Recycling. vol. 4 of Environmental Chemistry for a Sustainable World, pp. 321–349.
ATSDR (1999) Toxicological Profile of Lead, US. Department of Health and Human Services Agency for Toxic Substances and Disease Registry, Washington, DC, USA.
Conrad ME, Barton JC (1978) Factors affecting the absorption and excretion of lead in the rat. Gastroenterol. 74: 731-740. doi: https://doi.org/10.1016/0016-5085(78)90253-6.
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr. Med. Chem. 12: 1161–1208. doi: 10.2174/0929867053764635.
Sugawara E, Nakamura K, Miyake T, Fukumura A, Seki Y (1991) Lipid peroxidation and concentration of glutathione in erythrocytes from workers exposed to lead. Br. J. Ind. Med. 48: 239–242.doi: 10.1136/oem.48.4.239.
Ahamed M, Verma S, Kumar A, Siddiqui M (2005) Environmental exposure to lead and its correlation with biochemical indices in children. Sci. Total Environ. 346: 48–55. doi: 10.1016/j.scitotenv.2004.12.019.
Yiin SJ, Lin TH (1995) Lead-catalyzed peroxidation of essential unsaturated fatty acid. Biol. Trace Elem. Res. 50: 167-172. doi: 10.1007/bf02789419.
IARC (1993) International Agency for research on Cancer, Beryllium, Cadmium. Mercury and exposures in the glass manufacturing industry, vol. 58, International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Human (IARC), Lyon, France.
Shimada H, Yasutake A, Hirashima T, Takamure Y, Kitano T, Waalkes MP, Imamura Y (2008) Strain difference of cadmium accumulation by liver slices of inbred Wistar- Imamichi and Fischer 344 rats. Toxicol. in Vitro 22: 338–343. doi: 10.1016/j.tiv. 2007.09.013.
Wang Y, Fang J, Leonard SS, Rao KMK (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 36: 1434–1443. https://doi.org/10.1016/j.freeradbiomed.2004.03.010.
López E, Arce C, Oset-Gasque MJ, Ca˜nadas C, Gonz´alez MP (2006) Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic. Biol. Med. 40: 940–951. https://doi.org/10.1016/j.freeradbiomed. 2005.10.062.
Kondoh M, Araragi S, Sato K, Higashimoto M, Takiguchi M, Sato M (2002) Cadmium induces apoptosis partly via caspase-9 activation in HL-60 cells. Toxicology 17: 111– 117.
Clifton II JC (2007) Mercury exposure and public health. Pediatr. Clin. Nor. Am. 54: 237–269. doi: 10.1016/j.pcl.2007.02.005.
Langford NJ, Ferner RE (1999) Toxicity of mercury. J. Hum. Hypertens. 13: 651–656. https://doi.org/10.1038/sj.jhh.1000896.
Hultberg B, Anderson A, Isaksson A (2001) Interaction of metals and thiols in cell damage and glutathione distribution: potentiation of mercury toxicity by dithiothreitol. Toxicol. 156: 93-100.
Barnes JL, McDowell EM, McNeil JS (1980) Studies on the pathophysiology of acute renal failure. V. Effect of chronic saline loading on the progression of proximal tubular injury and functional impairment following administration of mercuric chloride in the rat. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 32: 233–260.
Siu SS, Yeung JH, Lau TK (2000) A study on placental transfer of diclofenac in first trimester of human pregnancy. Hum. Reprod. 15: 2423-2425. doi: 10.1093/humrep/15.11.2423
Kudo C, Kori M, Matsuzaki K, Yamai K, Nakajima A, Shibuya A, Niwa H, Kamisaki Y, Wada K (2003) Diclofenac inhibits proliferation and differentiation of neural stem cells. Biochem. Pharmacol. 66: 289-295. https://doi.org/10.1016/S0006-2952(03)00235-1.
Garner A (1992) Adaptation in the pharmaceutical industry, with particular reference to gastrointestinal damages and diseases. Scand. J. Gastroenterol. 27: 83-89.
Ragbetli MC, Ozyurt B, Aslan H, Odaci E, Gokcimen A, Sahin B, Kaplan S (2007) Effect of prenatal exposure to diclofenac sodium on Purkinje cell numbers in rat cerebellum: a stereological study. Brain Res. 1174: 130-135. doi: 10.1016/j.brainres. 2007.08.025.
Ennis ZN, Dideriksen D, Vaegter HB, Handberg G, Pottegard A (2016) Acetaminophen for chronic pain: a systematic review on efficacy. Basic Clin. Pharmacol. Toxicol. 118: 184-189. doi: 10.1111/bcpt.12527. doi: 10.1111/bcpt.12527.
Bjarnason I, Scarpignato C, Takeuchi K, Rainsford KD (2007) Determinants of the short-term gastric damage caused by NSAIDs in man. Aliment. Pharmacol. Ther. 26: 95–106. doi: 10.1111/j.1365-2036.2007.03348.x.
Awtry EH, Loscalzo J (2000) Cardiovascular drugs. aspirin. Circulation 101: 1206-1218.
García Rodríguez LA, Jick H (1994) Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 343: 769-772. https://doi.org/10.1016/S0140-6736(94)91843-0.
Yoshikawa T, Naito Y, Kishi A, Tomii T, Kaneko T, Jinuma S, Ichikawa H, Yasuda M, Takahashi S, Kondo M (1993) Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 34: 732-737. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1374252.
Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Bielanski W, Brzozowska I, Stachura J, Han EG (1997) The role of melatonin and L-tryptophan in prevention of acute gastric lesions induced by stress, ethanol, ischemia, and aspirin. J. Pineal Res. 23: 79-89. https://doi.org/10.1111/j.1600-079X.1997.tb00339.x.
Banerjee RK (1990) Nonsteroidal anti-inflammatory drugs inhibit gastric peroxidase activity. Biochim. Biophys. Acta 1034: 275-280.
Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK (1997) Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic. Biol. Med. 23: 8-18. https://doi.org/10.1016/S0891-5849(96)00547-3.
Naito Y, Yoshikawa T (2006) Oxidative stress Involvement and gene expression in indomethacin-induced gastropathy. Redox Rep. 11: 243–253. doi: 10.1179/135100006X 155021.
Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK (2006) Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidise inactivation and scavenging reactive oxygen. Free Radic. Biol. Med. 40: 1397–1408. doi: 10.1016/ j.freeradbiomed.2005.12.016.
Mei Q, Diao L, Xu J, Liu X, Jin J (2011) A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta Pharmacol. Sin. 32: 495–502. doi: 10.1038/aps.2010.225.
Phull PS, Green CJ, Jacyna MR (1995) A radical view of the stomach: the role of oxygen-derived free radicals and anti-oxidants in gastroduodenal disease. Eur. J. Gastroenterol. Hepatol. 7: 265–274.
Mallick IH, Yang W, Winslet MC, Seifalian AM (2004) Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig. Dis. Sci. 49: 1359–1377.
Benassi B, Fanciulli M, Fiorentino F, Porrello A, Chiorino G, Loda M, ZupiG,Biroccio A (2006) c-Mycphosphorylation is required for cellular response to oxidative stress. Mol. Cell 21: 509–519. doi: 10.1016/j.molcel.2006.01.009.
Sasaki M, Joh T (2007) Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant, protective agents. J. Clin. Biochem. Nutr. 40: 1–12. doi: 10.3164/jcbn.40.1.
Heslin MJ, Hawkins A, Boedefeld W, Arnoletti JP, Frolov A, Soong R, Urist MM, Bland KI (2005) Tumor-associated down-regulation of 15-lipoxygenase-1 is reversed by celecoxib in colorectal cancer. Ann. Surg. 241: 941–946. doi: 10.1097/01.sla.0000164177.95620.c1.
Jarry A, Bach-Ngohou K, Masson D, Dejoie T, Lehur PA, Mosnier JF, Denis MG, Laboisse CL (2006) Human colonic myocytes are involved in post-ischemic inflammation through ADAM17-dependent TNF alpha production. Br. J. Pharmacol. 147: 64–72. doi: 10.1038/sj.bjp.0706449.
Nilsson UA, Lundgren O, Haglind E, Bylund-Fellenius AC (1989) Radical production during in vivo intestinal ischemia and reperfusion in the cat. Am. J. Physiol. 257: G409–G414.
Jaeschke H, Mitchell JR (1989) Mitochondria and xanthine oxidase both generate reactive oxygen species in isolated perfused rat liver after hypoxic injury. Biochem. Biophys. Res. Commun. 160: 140–147.
Zhang J, Piantadosi CA (1992) Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J. Clin. Invest. 90: 1193–1199.
Bindoli A, Cavallini L, Rigobello MP, Coassin M, Di Lisa F (1988) Modification of the xanthine-converting enzymeof perfused rat heart during ischemia and oxidative stress. Free Radic. Biol. Med. 4: 163–167.
Liu JQ, Zelko IN, Folz RJ (2004) Reoxygenation-induced constriction in murine coronary arteries: the role of endothelial NADPH oxidase (gp91phox) and intra cellular superoxide. J. Biol. Chem. 279: 24493–24497.DOI: 10.1074/jbc.M402920200.
Grisham MB, Granger DN (1994) Metabolic sources of reactive oxygen metabolites during oxidant stress and ischemia with reperfusion. Clin. Chest Med. 10: 71–81.
Grace PA (1994) Ischaemia-reperfusion injury. Br. J. Surg. 81: 637–647.
Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L (2013) Obesity-associated oxidative stress: strategies finalized to improve redox state. Int. J. Mol. Sci. 21: 10497-538. doi: 10.3390/ijms140510497.
Auberval N, Dal S, Bietiger W, Pinget M, Jeandidier N, Maillard-Pedracini E, Valérie SK, Séverine S (2014) Metabolic and oxidative stress markers in wistar rats after 2 months on a high-fat diet. Diabetol. Metab. Syndr. 6: 130. doi: 10.1186/1758-5996-6-130.
Vargas-Robles H, Rios A, Arellano-Mendoza M, Escalante BA, Schnoor M (2015) Antioxidative diet supplementation reverses high-fat diet-induced increases of cardiovascular risk factors in mice. Oxid. Med. Cell Longev. 15: 1-9. doi: 10.1155/2015 /467471.
de La Serre CB, Ellis CL, Lee J, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE (2010) Propensity to high-fat diet induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G440–G448. doi: 10.1152/ajpgi.00098.2010.
Kolios G, Valatas V, Ward SG (2004) Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunol. 113: 427–437. doi: 10.1111/j.1365 -2567.2004.01984.x.
Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66. doi: https://doi.org/10.32794/ mr11250011.
Pessayre D, Mansouri A, Fromenty B (2002) Nonalcoholic steatosis and steatohepatitis. Mitochondrial dysfunction in steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 282: G193-G199. doi: 10.1152/ajpgi.00426.2001.
Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S (2008) Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metab. 57: 1071-1077. doi: 10.1016/j.metabol.2008.03.010.
McManus IR, Contag AO, Olson RE (1966) Studies on the identification and origin of ethanol in mammalian tissues. J. Biol. Chem. 241: 349–356.
Lester D, Keokosky WZ, Felzenberg F (1968) Effect of pyrazoles and other compounds on alcohol metabolism. Q. J. Stud. Alcohol 29: 449–454.
Schnabl B, Brenner DA (2014) Interactions between the intestinal microbiome and liver diseases. Gastroenterol. 146: 1513–1524. doi: 10.1053/j.gastro.2014.01.020.
Pronko P, Bardina L, Satanovskaya V, Kuzmich A, Zimatkin S (2002) Effect of chronic alcohol consumption on the ethanol-and acetaldehyde-metabolizing systems in the rat gastrointestinal tract. Alcohol Alcohol. 37: 229–235. https://doi.org/10.1093 /alcalc/37.3.229.
Vucevic D, Mladenovic D, Ninkovic M, Stankovic MN, Jorgacevic B, Stankovic MS, de Luka S, Radosavljevic T (2013). Influence of aging on ethanol-induced oxidative stress in digestive tract of rats. Hum. Exp. Toxicol. 32: 698. doi: 10.1177/0960327112467045.
Wild CP, Hardie LJ (2003) Reflux, Barrett’s oesophagus and adenocarcinoma: burning questions. Nat. Rev. Cancer 3: 676–684.
Song S, Guha S,Liu K, Buttar NS, Bresalier RS (2007) COX-2 induction by unconjugated bile acids involves reactive oxygen species-mediated signalling pathways in Barrett’s oesophagus and oesophageal adenocarcinoma. Gut 56: 1512–1521.doi: 10.1136/gut.2007.121244
Diakowska D, Krzystek KM, Lewandowski A, Grabowski K, Diakowski W (2008) Evaluation of 8-hydroxy deoxyguanosine, thiobarbituric acid-reactive substances and total antioxidant status as possible disease markers in oesophageal malignancies. Clin. Biochem. 41: 796–803. DOI: 10.1016/j.clinbiochem.2008.03.014.
O’Connor HJ, Schorah CJ, Habibzedah N, Axon AT, Cockel R (1989) Vitamin C in the human stomach: relation to gastric pH, gastroduodenal disease, and possible sources. Gut 30: 436–442.
Naito Y, Yoshikawa T, Ando T, Kishi A, Ueda S, Oyamada H, Kondo M (1992) Changes in superoxide dismutase activity in the gastric mucosa of peptic ulcer patients. J. Clin. Gastroenterol. 14: Suppl1: S131–S134.
Teshima S, Tsunawaki S, Rokutan K (1999) Helicobacter pylori lipopolysaccharide enhances the expression of NADPH oxidase components in cultured guinea pig gastric mucosal cells. FEBS Lett. 452: 243–246.
O’Hara AM, Bhattacharya A, Bai J, Mifflin RC, Smith MF Jr, Ryan KA, Scott KGE, Naganuma M, Casola A, Izumi T, Mitra S, Ernst PB, Crowe SE (2006) Interleukin-8 induction by Helicobacter pylori in human gastric epithelial cells is dependent on a purinic/ apyrimidinic endonuclease-1/redoxfactor-1. J. Immunol. 177: 7990–7999.
Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, Patel J, Kramer B, Boldogh I, Ernst PB, Crowe SE (2007) Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect. Immun. 75: 4030–4039. doi: 10.1128/IAI.00172-07.
Bhattacharya S, Mathew G, Jayne DG, Pelengaris S, Khan M (2009) 15-Lipoxygenase-1in colorectal cancer: a review. Tumour Biol. 30: 185–199. doi: 10.1159/000236864.
Davies GR, Simmonds NJ, Stevens TR, Grandison A, Blake DR, Rampton DS (1992) Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut 33: 1467–1472. doi: 10.1136/gut.33.11.1467.
Jung HK, Lee KE, Chu SH, Yi SY (2001) Reactive oxygen species activity, mucosal lipoper-oxidation and glutathione in Helicobacter pylori-infected gastric mucosa. J. Gastroenterol. Hepatol. 16: 1336–1340.
O’Connor PM, Lapointe TK, Beck PL, Buret AG (2010) Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm. Bowel Dis. 16: 1411–1420. doi: 10.1002/ibd.21217.
Colgan SP, Taylor CT (2010) Hypoxia: anal arm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7: 281–287.doi: 10.1038/nrgastro.2010.39.
Iborra M, Moret I, Rausell F, Bastida G, Aguas M, Cerrillo E, Nos P, Beltran B (2011) Role of oxidative stress and antioxidant enzymes in Crohn’s disease. Biochem. Soc. Trans 39: 1102–1106. doi: 10.1042/BST0391102.
Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS (2007) Commensal bacteria modulate cullin-dependent signalling via generation of reactive oxygen species. EMBO J. 26: 4457–4466. doi: 10.1038/sj.emboj.7601867.
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Bio. Med. Res. Int. Article ID 640754, 26 pages.http://dx.doi.org/10.1155/2014/640754
Hooverand TD, Aposhian HV (1983) BAL increases the arsenic-74 content of rabbit brain. Toxicol. Appl. Pharm. 70: 160–162.
Risher JF, Amler SN (2005) Mercury exposure: evaluation and intervention. The inappropriate use of chelating agents in the diagnosis and treatment of putative mercury poisoning. Neuro. Toxicol. 26: 691–699.doi: 10.1016/j.neuro.2005.05.004.
ChisolmJr JJ, Thomas DJ (1985) Use of 2, 3-dimercaptopropane-1-sulfonatein treatment of lead poisoning in children. J. Pharmacol. Exp. Ther. 235: 665–669.
Zalups RK, Parks LD, Cannon VT, Barfuss DW (1998) Mechanisms of action of 2,3-dimercaptopropane-1-ulfonate and the transport, disposition, and toxicity of inorganic mer-curyinisolated perfused segments of rabbit proximal tubules. Molecul. Pharmacol. 54: 353–363.
Mayo JC, Tan DX, Sainz RM, Natarajan M, LopezBurillo S, Reiter RJ (2003) Protection against oxidative protein damage induced by metal catalyzed reaction or alkyl peroxyl radicals: comparative effects of melatonin and other antioxidants. Biochim. Biophys. Acta 1620: 139–150.
Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH (1998) 𝛼-lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 24: 1023–1039.
Sumathi R, Baskaran G, Varalakshmi P (1996) Effect of DL𝛼-lipoic acid on tissue redox state in acute cadmium-challenged tissues. J. Nutr. Biochem. 7: 85–92.
Muller L (1989) Protective effects of DL-𝛼-lipoicacid on cadmium induced deterioration of the rat’s patocytes. Toxicol. 58: 175–185.
Young IS, Woodside JV (2001) Antioxidants in health and disease. J. Clin. Pathol. 54: 176–186.
Rendon-Ramirez A, Cerbon-Solorzano J, Maldonado-Vega M, Quintanar-Escorza MA, Calderon-Salinas JV (2007) Vitamin-E reduces the oxidative damage on delta-aminolevulinic dehydratase induced by lead intoxication in rat erythrocytes. Toxicol. In Vitro 21: 1121–1126. doi: 10.1016/j.tiv.2007.04.019.
Tandon SK, Singh S, Dhawan M (1992) Preventive effect of vitaminE in cadmium intoxication. Biomed. Environ. Sci. 5: 39–45.
Quig D (1998) Cysteine metabolism and metal toxicity. Altern. Med. Rev.3: 262–270.
Shaikh ZA, Zaman K, Tang W, Vu T (1999) Treatment of chronic cadmium nephrotoxicity by N-acetylcysteine. Toxicol. Lett. 104: 137–142.
Santra A, Chowdhury A, Ghatak S, Biswas A, Dhali GK (2007) Arsenic induces apoptosis in mouse liver is mitochondria dependent and isabrogated by N-acetylcysteine. Toxicol. Applied Pharmacol. 220: 146–155.doi: 10.1016/j.taap.2006.12.029.
Mitra E, Ghosh AK, Ghosh D, Mukherjee D, Chattopadhyay A, Dutta S, Pattari S K and Bandyopadhyay D (2012) Protective effect of aqueous curry leaf (Murraya koenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 50: 1340–1353. doi: 10.1016/j.fct.2012.01.048.
Mitra E, Ghosh AK, Ghosh D, Firdaus SB, Mukherjee D, Chattopadhyay A, Pattari SK, Datta S, Bandyopadhyay D (2014) Ameliorative effect of aqueous tulsi leaf (Ocimum sanctum) extract against cadmium-induced oxidative stress in rat liver. Int. J. Pharm. Pharm. Sci. 5: 557-568.
Graham DY (1990) The relationship between nonsteroidal anti-inflammatory drug use and peptic ulcer disease. Gastroenterol. Clin. Nor. Am. 19: 171–182.
Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I (2011) Analgesic effects of melatonin: A review of current evidence from experimental and clinical studies. J. Pineal Res. 51: 270–277. doi: 10.1111/j.1600-079x.2011.00895.x.
Lopez-Belmonte J, Whittle BJW, Moncada S (1993) The actions of nitric oxide donors in the prevention or induction of injury to the rat gastric mucosa. Br. J. Phamacol. 108: 73–78.
Reuter BK, Cirino G, Walkce JL (1994) Markedly reduced intestinal toxicity of a diclofenac derivative. Life Sci. 55: PL1-PL8. https://doi.org/10.1016/0024-3205 (94)90083-3.
Wallace JL (2006) Nitricoxide, aspirin-triggered lipoxins and NO-aspirinin gastric protection. Inflamm. Allergy Drug Targets 5: 133–137. doi: 10.2174/187152806776383116.
Soll AH, Weinstein WM, Kurata J, McCarthy D (1991) Nonsteroidal anti-inflammatory drugs and peptic ulcer disease. Ann. Intern. Med. 114: 387–319. doi: 10.18773/austprescr.2017.037.
Gavalas A, Hadjipetrou L, Kourounakis P (1998) Synthesis of novel derivatives of aroyl-aminoalcohols and 3-amino-substituted 1-phenylpropanols with potential anti-inflammatory and immunomodulating activity. J. Pharm. Pharmacol. 50: 583–591. https://doi.org/10.1111/j.2042-7158.1998.tb06891.x.
Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B, Gimona A, Matchaba P, Hawkey CJ, Chesebro JH (2004) Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 364: 675–684. doi: 10.1016/s0140-6736(04)16894-3.
Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59: 403–419. doi: 10.1111/jpi.12267.
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253–278. doi: 10.1111/jpi.12360.
Galano A, Tan DX, Reiter RJ (2018) Melatonin: a versatile protector against oxidative DNA damage. Molecules. 27: 23. doi: 10.3390/molecules23030530.
Konturek PC, Konturek SJ, Brzozowski T, Dembinski A, Zembala M, Mytar B, Hahn EG (1997) Gastroprotective activity of melatonin and its precursor, L-tryptophan, against stress-induced and ischemia-induced lesions is mediated by scavenge of oxygen radicals. Scand. J. Gastroenterol. 32: 433–438.
Martín M, Macías M, León J, Escames G, Khaldy H, Acuña- Castroviejo D (2002) Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell. Biol. 34: 348–357.
Ercan F, Cetinel S, Contuk G, Cikler E, Sener G (2004) Role of melatonin in reducing water avoidance stress induced degeneration of the gastrointestinal mucosa. J. Pineal Res. 37: 113–121. doi: 10.1111/j.1600-079X.2004.00143.x.
Srinivasan V, Lauterbach EC, Ho KY, Acuña-Castroviejo D, Zakaria R, Brzezinski A (2012) Melatonin in antinociception: Its therapeutic applications. Curr. Neuropharmacol. 10: 167–178. doi: 10.2174/157015912800604489.
Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C (1994) Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 269: 28531–28534.
Witt-Enderby PA, Radio NM, Doctor JS, Davis VL (2006) Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 41: 297–305. doi: 10.1111/j.1600-079x.2006.00369.x.
Boutin JA, Audinot V, Ferry G, Delagrange P (2005) Molecular tools to study melatonin pathways and actions. Trends Pharmacol. Sci. 26: 412–419. doi:10.1016/j.tips. 2005.06.006.
Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181-97. https://www.ncbi.nlm.nih.gov/pubmed/11899100.
Reiter RJ, Tan DX, Manchester LC, Qi W. (2001) Biochemical reactivity of melatonin with reactive oxygen and reactive nitrogen species: A review of the evidence. Cell. Biochem. Biophys. 34: 237–256. doi: 10.1385/CBB:34:2:237.
Cuzzocrea S, Reiter JR (2002) Pharmacological action of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2:153–165.
Nosál'ová V, Zeman M, Černá S, Navarová J, Zakálová M (2007) Protective effect of melatonin in acetic acid induced colitis in rats. J. Pineal Res. 42: 364–370. doi: 10.1111/j.1600-079X.2007.00428.x.
Torres-Farfan C, Richter HG, Rojas-García P, Vergara M, Forcelledo ML, Valladares LE, Torrealba F, Valenzuela GJ, Seron- Ferre M. (2003) mt1 Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 88: 450–458. doi: 10.1210/jc.2002-021048.
Saito S, Tachibana T, Choi YH, Denbow DM, Furuse M (2005) ICV melatonin reduces stress responses in neonatal chicks. Behav. Brain Res. 165: 197–203. doi: 10.1016/j.bbr.2005.06.045.
Lopez-Burillo S, Tan DX, Rodriguez-Gallego V, Manchester LC, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin and its derivatives cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5- methoxykynuramine, and 6-hydroxymelatonin reduced oxidative damage induced by Fenton reagents. J. Pineal Res. 34: 237–256. doi: 10.1034/j.1600-079x.2003.00025.x.
Poeggeler B, Reiter RJ, Hardeland R, Sewerynek E, Melchiorri D, Barlow-Walden LR (1995) Melatonin, a mediator of electron transfer and repair reactions acts synergistically with the chain breaking antioxidants ascorbate trolox and glutathione. Neuroendocrinol. Lett. 17: 87-92.
Raikhlin NT, Kvetnoy IM (1974) Lightening effect of the extract of human appendix mucosa on frog skin melanophores. Bull. Exp. Biol. Med. 8: 114–116.
Vakkuri O, Rintamaki H, Leppaluoto J (1985a) Presence of immunoreactive melatonin in different tissues of the pigeon. Gen. Comp. Endocrinol. 58: 69–75.
Vakkuri O, Rintamaki H, Leppaluoto J (1985b) Plasma and tissue concentrations of melatonin after midnight light exposure and pinealectomy in the pigeon. J. Endocrinol. 105: 263–268.
Huether G (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49: 665–670.
Lee PPN, Shiu SYU, Chow PH, Pang SF (1995) Regional and diurnal studies on melatonin binding sites in the duck gastrointestinal tract. Biol. Signals 4: 212–224.
Menendez-Pelaez A, Buzzell GR (1992) Harderian gland indoles. In: Webb SM, Hoffman RA, Puig-Domingo ML, Reiter RJ (eds) Harderian glands: Porphyrin metabolism, behavioral, and endocrine effects. Springer, Berlin, pp. 219–234.
Chow PH, Lee PN, Poon AMS, Shiu SYW, Pang SF (1996) The gastrointestinal system: A site of melatonin paracrine action. In: Tang PL, Pang SF, Reiter RJ (eds.) Melatonin: A universal photoperiodic signal with diverse action. Front. Horm. Res. Basel, Karger, 21: 123–132.
Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni GJM (2000) Evidence of melatonin synthesis in mouse and human bone marrow. J. Pineal Res. 28: 193–202.
Fu Z, Kato H, Kotera N, Noguchi T, Sugahara K, Kubo T (2001) Regulation of hydroxyindole-O-methyltransferase gene expression in Japanese quail (Coturnixcoturnix japonica). Biosci. Biotechnol. Biochem. 65: 2504–2511. doi: 10.1271/bbb.65.2504.
Stefulj J, Hörtner M, Ghosh M, Schauenstein K, Rinner I, Wölfler A, Semmler J, Liebmann PM (2001) Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 30: 243–247.
Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R (2008) Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab.19: 17–24.
Hong GX, Pang SF (1995) N-Acetyltransferase activity in the quail (Cotornixcoturnixjap) duodenum. Comp. Biochem. Physiol. 112: 251–255.
Lovenberg W, Jequier E, Sjoerdsma A (1967) Tryptophan hydroxylation: Measurement in pineal gland, brain stem and carcinoid tumor. Science 155: 217–219.
Balemans MGM, Bary FAM, Legerstee WC, van Benthem J (1978) Estimation of the methylating capacity in the pineal gland of the rat with special reference to the methylation of N-acetylserotonin and 5-hydroxytryptophol separately. Experientia 34: 1434–1435.
Voisin P, Namboodiri MAA, Klein DC (1984) Arylamine N-acetyltransferase and arylalkylamine N-acetyltransferase in the mammalian pineal gland. J. Biol. Chem. 259: 10913–10918.
Axelrod J, Weissbach H. (1960) Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 131: 1312–1312.
León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38: 1–9. doi: 10.1111/j.1600-079x.2004.00181.x.
Xu S, Pi H, Zhang L, Zhang N1, Li Y, Zhang H, Tang J, Li H, Feng M, Deng P, Guo P, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Wang W, Reiter RJ, Yu Z, Zhou Z (2016) Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J Pineal Res. 60: 291-302. doi: 10.1111/jpi.12310.
Li M, Pi H, Yang Z, Reiter RJ, Xu S, Chen X, Chen C1, Zhang L, Yang M, Li Y, Guo P1, Li G, Tu M, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Yu Z, Zhou Z (2016) Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J. Pineal Res. 61: 353-69. doi: 10.1111/jpi.12353.
Sener G, Sehirli AO, Ayanoglu-Dülger G (2003) Melatonin protects against mercury (II)-induced oxidative tissue damage in rats. Pharmacol. Toxicol. 93: 290-296.
Zhang Y, Wei Z, Liu W, Wang J, He X, Huang H, Zhang J, Yang Z (2017) Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget. 8: 3773–3780. doi: 10.18632/oncotarget.13931.
Mishra S, Ghosh D, Dutta M, Chattopadhyay A, Bandyopadhyay D (2013) Melatonin protects against lead-induced oxidative stress in stomach,duodenum and spleen of male Wistar rats. J. Pharm. Res. 1: 997-1004.
Cabeza J, Alarcón-de-la-Lastra C, Jiménez D, Martín MJ, Motilva V (2003) Melatonin modulates the effects of gastric injury in rats: role of prostaglandins and nitric oxide. Neurosignals 12: 71–77.doi: 10.1159/000071816.
Ozturk H, Oztürk H, Yagmur Y, Uzunlar AK (2006) Effects of melatonin administration on intestinal adaptive response after massive bowel resection in rats. Dig. Dis. Sci. 51: 333–337. doi: 10.1007/s10620-006-3134-y.
Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: Nature's most versatile biological signal? FEBS J. 273: 2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x.
Konturek PC, Konturek SJ, Burnat G, Brzozowski T, Brzozowska I, Reiter RJ (2008) Dynamic physiological and molecular changes in gastric ulcer healing achieved by melatonin and its precursor L-tryptophan in rats. J. Pineal Res. 45: 180–190. doi: 10.1111/j.1600-079x.2008.00574.x.
Hardeland R, Tan DX, Reiter RJ (2009) Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 47: 109–124. doi: 10.1111/j.1600-079x.2009.00701.x.
Gitto E, Pellegrino S, Gitto P, Barberi I, Reiter RJ (2009) Oxidative stress of the newborn in the pre-and postnatal period and the clinical utility of melatonin. J. Pineal Res. 44: 128–139. doi: 10.1111/j.1600-079x.2008.00649.x.
Chen LJ, Gao YQ, Li XJ, Shen DH, Sun FY (2005) Melatonin protects against MPTP/MPP+ -induced mitochondrial DNA oxidative damage in vivo and in vitro. J. Pineal Res. 39: 34–42. doi: 10.1111/j.1600-079x.2005.00209.x.
Acuña-Castroviejo D, Escames G, León J, Carazo A, Khaldy H (2003) Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 527: 549–557.
Hussein MR, Abu-Dief EE, Abd El-Reheem MH, Abd-Elrahman A (2005) Ultrastructural evaluation of the radioprotective effects of melatonin against X-ray-induced skin damage in Albino rats. Int. J. Exp. Pathol. 86: 45–55. doi: 10.1111/j.0959-9673.2005.00412.x.
Sener G, Sert G, Sehirli AO, Arbak S, Gedik N, Ayanoglu-Dülger G (2006) Melatonin protects against pressure ulcer-induced oxidative injury of the skin and remote organs in rats. J. Pineal Res. 40: 280–287. doi: 10.1111/j.1600-079x.2005.00313.x.
Jaworek J, Brzozowski T, Konturek SJ (2005) Melatonin as an organoprotector in the stomach and the pancreas. J. Pineal Res. 38: 73–83. doi: 10.1111/j.1600-079x.2004.00179.x.
Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36: 195–203. https://doi.org/10.1111/j.1600-079X.2004.00118.x.
Ates B, Yilmaz I, Geckil H, Iraz M, Birincioglu M, Fiskin K (2004) Protective role of melatonin given either before ischemia or prior to reperfusion on intestinal ischemia-reperfusion damage. J. Pineal Res. 37: 149–152.doi: 10.1111/j.1600-079X.2004.00148.x.
Kazez A, Demirbag M, Ustundag B, Ozercan IH, Saglam M (2000) The role of melatonin in prevention of intestinal ischemia-reperfusion injury in rats. J. Pediatr. Surg. 35: 1444–1448.
Ustundag B, Kazez A, Demirbag M, Canatan H, Halifeoglu I, and Ozercan IH (2000) Protective effect of melatonin on antioxidative system in experimental ischemia-reperfusion of rat small intestine. Cell Physiol. Biochem.10: 229–236. DOI: 10.1159/000016354.
Sileri P, Sica GS, Gentileschi P, Venza M, Benavoli D, Jarzembowski T, Manzelli A, Gaspari AL (2004) Melatonin reduces bacterial translocation after intestinal ischemia-reperfusion injury. Transplant Proc. 36: 2944–2946.doi: 10.1016/j.transproceed. 2004.10.085.
Ozacmak VH, Sayan H, Arslan SO, Altaner S, Aktas RG (2005) Protective effect of melatonin on contractile activity and oxidative injury induced by ischemia and reperfusion of rat ileum. Life Sci. 76: 1575–1588. doi: 10.1016/j.lfs.2004.08.031.
Ganguly K, Maity P, Reiter RJ, Swarnakar S (2005) Effect of melatonin on secreted and induced matrix metalloproteinase -9 and -2 activity during prevention of indomethacin-induced gastric ulcer. J. Pineal Res. 39: 307–315. doi: 10.1111/j.1600-079x.2005.00250.x.
Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S (2006) Hydrogen peroxide-mediated down regulation of matrixmetalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic. Biol. Med. 41: 911–925. https://doi.org/10.1016/j.freeradbiomed.2006.04.022.
Brzozowska I, Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Pajdo R, Drozdowicz D, Pawlik M, Ptak A, Hahn EG (2002) Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J. Pineal Res. 32: 149–162. https://doi.org/10.1034/j.1600-079x.2002.1o811.x.
Brzozowski T, Konturek PC, ZwirskaKorczala K, Konturek SJ, Brzozowska I, Drozdowicz D, Sliwowski Z, Pawlik M, Pawlik WW, Hahn EG (2005) Importance of the pineal gland, endogenous prostaglandins and sensory nerves in the gastroprotective actions of central and peripheral melatonin against stress-induced damage. J. Pineal Res. 39: 375–385. https://doi.org/10.1111/j.1600-079X.2005.00264.x.
Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int. J. Mol. Sci.14: 8638–8683. doi: 10.3390/ijms14048638.
Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX (2000) Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 917: 376–386. https://doi.org/10.1111/j.1749-6632.2000.tb05402.x.
Cuzzocrea S, Reiter RJ (2001) Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur. J. Pharmacol. 426: 1–10. https://doi.org/10.1016/S0014-2999(01)01175-X.
Lissoni P, Rovelli F, MeregalliS, Fumagalli L, Musco F, Brivio F, Brivio O, Esposti G (1997) Melatonin as a new possible anti-inflammatory agent. J. Biol. Regul. Homeost Agents 11: 157–159.
Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, Marchal JA, Adem A, Fernández-Vázquez G (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal R. 54: 381–388. doi: 10.1111/jpi.12012.
Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J (2013) A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J. Pineal Res. 54: 1–14.doi: 10.1111/j.1600-079X.2012.01014.x.
Dong Y, Fan C, Hu W, Jiang S, Ma Z, Yan X, Deng C, Di S, Xin Z, Wu G, Yang Y, Reiter RJ, Liang G (2016) Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signalling. J. Pineal Res. 60: 253–262.doi: 10.1111/jpi.12300.
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51: 1–16. doi: 10.1111/j.1600-079X.2011.00916.x.
Baeuerle PA, Baltimore D (1996) NF-kappa B: ten years after. Cell 87: 13–20.
Li JH, Yu JP, Yu HG Xi-Ming Xu, Liang-Liang Yu, Jin Liu, He-Sheng Luo (2005) Melatonin reduces inflammatory injury through inhibiting NF-kappaB activation in rats with colitis. Mediators Inflamm. 2005: 185–193.doi: 10.1155/MI.2005.185.
Vriend J, Reiter RJ (2014) Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 115: 8–14.doi: 10.1016/j.lfs.2014.08.024.
Brzezinski A (1997) Melatonin in humans. N. Engl. J. Med. 336: 186–195. doi: 10.1056/NEJM199701163360306.
Volt H, García JA, Doerrier C, Díaz-Casado ME, Guerra-Librero A, López LC, Escames G, Tresguerres JA, Acuña-Castroviejo D (2016) Same molecule but different expression: aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 60: 193–205.doi: 10.1111/jpi.12303.
D’emmanueleDi Villa Bianca R, Marzocco S, Di Paola R, Autore G, Pinto A, Cuzzocrea S, Sorrentino R (2004) Melatonin prevents lipopolysaccharide-induced hypore activity in rat. J. Pineal Res. 36: 146–154.
Deng WG, Tang ST, Tseng HP, Wu KK (2006) Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 108: 518–524.doi: 10.1182/blood-2005-09-3691
Tamura EK, CeconE, Monteiro AW, Silva CL, Markus RP (2009) Melatonin inhibits LPS-induced NO production in rat endothelial cells. J. Pineal Res. 46: 268–274.doi: 10.1111/j.1600-079X.2008.00657.x.
This work is licensed under a Creative Commons Attribution 4.0 International License