Verification of agomelatine in comparison with melatonin as a therapeutic agent to treat breast cancer
Agomelatine and melatonin in breast cancer cells
Abstract
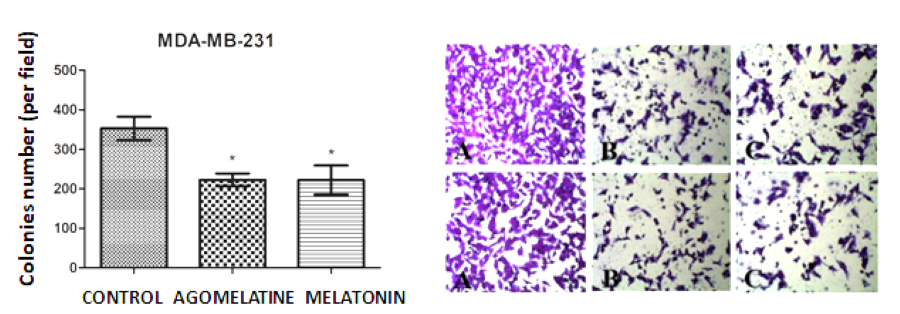
The breast cancer (BC) has a high rate of morbidity and mortality; thus, the discovery of new therapeutic targets is of great interest for researchers. Previous studies have documented that melatonin, the main hormone synthesized by the pineal gland, plays important roles in the control of breast tumorigenesis. Similar to melatonin, agomelatine, a melatonin analogue can also perform its functions by binding to G protein coupled melatonin membrane receptors MT1 and MT2. In a series of studies carried out in two breast cancer cell lines (MCF-7 and MDA-MB-231 strains), the dose-responsive curves have been identified regarding cell viability, clonogenic survival, and cell migration. The results indicate that agomelatine has the potential to reduce the proliferative capacity in both cell lines, while melatonin significantly reduced the proliferative rate of triple-negative BC cells. Notably, agomelatine and melatonin showed the same inhibitory effect on BC cell migration. Collectively, agomelatine treatment caused a greater reduction in BC cell growth than that of melatonin which only suppressed the proliferative capacity of triple-negative BC. Also, melatonin and agomelatine have the same inhibitory response to migratory capacity of triple-negative BC cells. Based on the results from current study on BC cells, agomelatine could be considered as a promising adjuvant therapeutic agent compared to melatonin for BC treatment.
References
2. National Cancer Institute (2018) Disponível em:
3. Harris E (2018) Precision medicine for breast cancer: The paths to truly individualized diagnosis and treatment. Int. J. Breast Cancer. 2018: 4809183.
4. Veiga ECA, Simões R, Valenti VE, et al. (2019) Repercussions of melatonin on the risk of breast cancer: a systematic review and meta-analysis. Rev. Assoc. Med. Bras. 65: 699-705.
5. Manchester C, Coto-Montes A, Boga JA, et al. (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59: 403-419.
6. Reiter R, Rosales-Corral SA, Tan D-X, et al. (2017) Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 18: 843.
7. Hill M, Belancio VP, Dauchy RT, et al. (2015) Melatonin: an inhibitor of breast cancer. Endocr. Relat. Cancer 22: R183-R204.
8. Nooshinfar E, Safaroghli-Azar A, Bashash D, et al. (2017) Melatonin, an inhibitory agent in breast cancer. Breast Cancer 24: 42-51.
9. Reiter RJ, Tan D-X, Korkmaz A, et al. (2007) Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit. Rev. Oncog. 13: 303-328.
10. Borin T, Arbab AS, Gelaleti GB, et al. (2016) Melatonin decreases breast cancer metastasis by modulating Rho‐associated kinase protein‐1 expression. J. Pineal Res. 60: 3-15.
11. Alonso-González C, Gonzáles A, Martínez-Campa C, et al. (2016) Melatonin enhancement of the radiosensitivity of human breast cancer cells is associated with the modulation of proteins involved in estrogen biosynthesis. Cancer Lett. 370: 145-152.
12. Kennedy S, Rizvi S (2010) Agomelatine in the treatment of major depressive disorder: potential for clinical effectiveness. CNS Drugs. 24: 479-499.
13. Gatti G, Lucini V, Dugnani S, et al. (2017) Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget 8: 68338.
14. De Bernardis D, Fornaro M, Serroni N, et al. (2015) Agomelatine beyond borders: current evidences of its efficacy in disorders other than major depression. Inter. J. Mol. Sci. 16: 1111-1130.
15. Arif I, Hooper CL, Greco F, et al. (2013) Increasing doxorubicin activity against breast cancer cells using PPARγ-ligands and by exploiting circadian rhythms: Reducing the cardiovascular toxicity of doxorubicin. Br. J. Pharmacol. 169: 1178-1188.
16. Liu J, Clough SJ, Hutchinson AJ, et al. (2018) MT1 and MT2 melatonin receptors: a therapeutic perspective. Ann. Rev. Pharmacol. Toxicol. 56: 361-383.
17. Emet M, Ozcan H, Ozel L, Yayla M, et al. (2016) A review of melatonin, its receptors and drugs. Eurasian J. Med. 48: 135-141.
18. Oprea-Ilies G, Haus E, Sackett-Lundeen L, et al. (2013) Expression of melatonin receptors in triple negative breast cancer (TNBC) in African American and Caucasian women: relation to survival. Breast Cancer Res. Treat. 137: 677-687.
19. Martínez-Campa C, Menéndez-Menéndez J, Gonzáles A, et al. (2017) What is known about melatonin, chemotherapy and altered gene expression in breast cancer. Oncol. Lett. 13: 2003-2014.
20. Kast R, Skulli N, Cos S, et al. (2017) The ABC7 regimen: A new approach to metastatic breast cancer using seven common drugs to inhibit epithelial-to- mesenchymal transition and augment capecitabine efficacy. Breast Cancer: Dove Med. Press 9: 495-514.
21. Bielecka-Wajdman A, Lesiak M, Ludyga T, et al. (2017) Reversing glioma malignancy: a new look at the role of antidepressant drugs as adjuvant therapy for glioblastoma multiform. Cancer Chemother. Pharmacol. 79: 1249-1256.
22. De Berardis D, Brucchi M, Serroni N, et al. (2014) Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin. Neuropharmacol. 37: 31-33.
23. Jardim-Perassi BV, Arbab AS, Ferreira LC, et al. (2014) Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS One 9: e85311.
24. Balça-Silva J, Neves SS, Gonçalves AC, et al. (2012) Effect of miR-34b overexpression on the radiosensitivity of non-small cell lung cancer cell lines. Anticancer Res. 32: 1603-1609.
25. Franken NA, Rodermond HM, Stap J, et al. (2006) Clonogenic assay of cells in vitro. Nat. Protoc. 1: 2315-2319.
26. Hevia D, González-Menédez P, Quiros-González, I, et al. (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58: 234-250.
27. Oliveira J, Marques JM, Lacerda JZ, et al. (2019). Melatonin down regulates microRNA-10a and decreases invasion and migration of triple-negative breast cancer cells. Melatonin Res. 2: 86-99.
28. Jablonska K, Pula B, Zemla A, et al. (2013). Expression of melatonin receptor MT 1 in cells of human invasive ductal breast carcinoma. J. Pineal Res. 54: 334-345.
29. Girgert, R, Hanf V, Emons G, et al. (2009). Membrane‐bound melatonin receptor MT1 down‐regulates estrogen responsive genes in breast cancer cells. J. Pineal Res. 47: 23-31.
30. Treeck, O, Haldar C, Ortmann O (2006) Antiestrogens modulate MT1 melatonin receptor expression in breast and ovarian cancer cell lines. Oncol. Rep. 15: 231-235.
31. Mao L, Yuan L, Xiang S, et al. (2014). Molecular deficiency (ies) in MT1 melatonin signaling pathway underlies the melatonin‐unresponsive phenotype in MDA‐MB‐231 human breast cancer cells. J. Pineal Res. 56: 246-253.
32. Liu J, Clough SJ, Hutchinson AJ, et al. (2016) MT1 and MT2 melatonin receptors: a therapeutic perspective. Ann. Rev. Pharmacol. Toxicol. 56: 361-383.
33. Comşa S, Cimpean M, Raica M (2015) The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 35: 3147-3154.
34. Taylor D, Sparshatt A, Varma S, et al. (2014) Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ 348: g1888.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


