Melatonin: a novel strategy for prevention of obesity and fat accumulation in peripheral organs through the improvements of circadian rhythms and antioxidative capacity
Melatonin alleviates obesity
Abstract
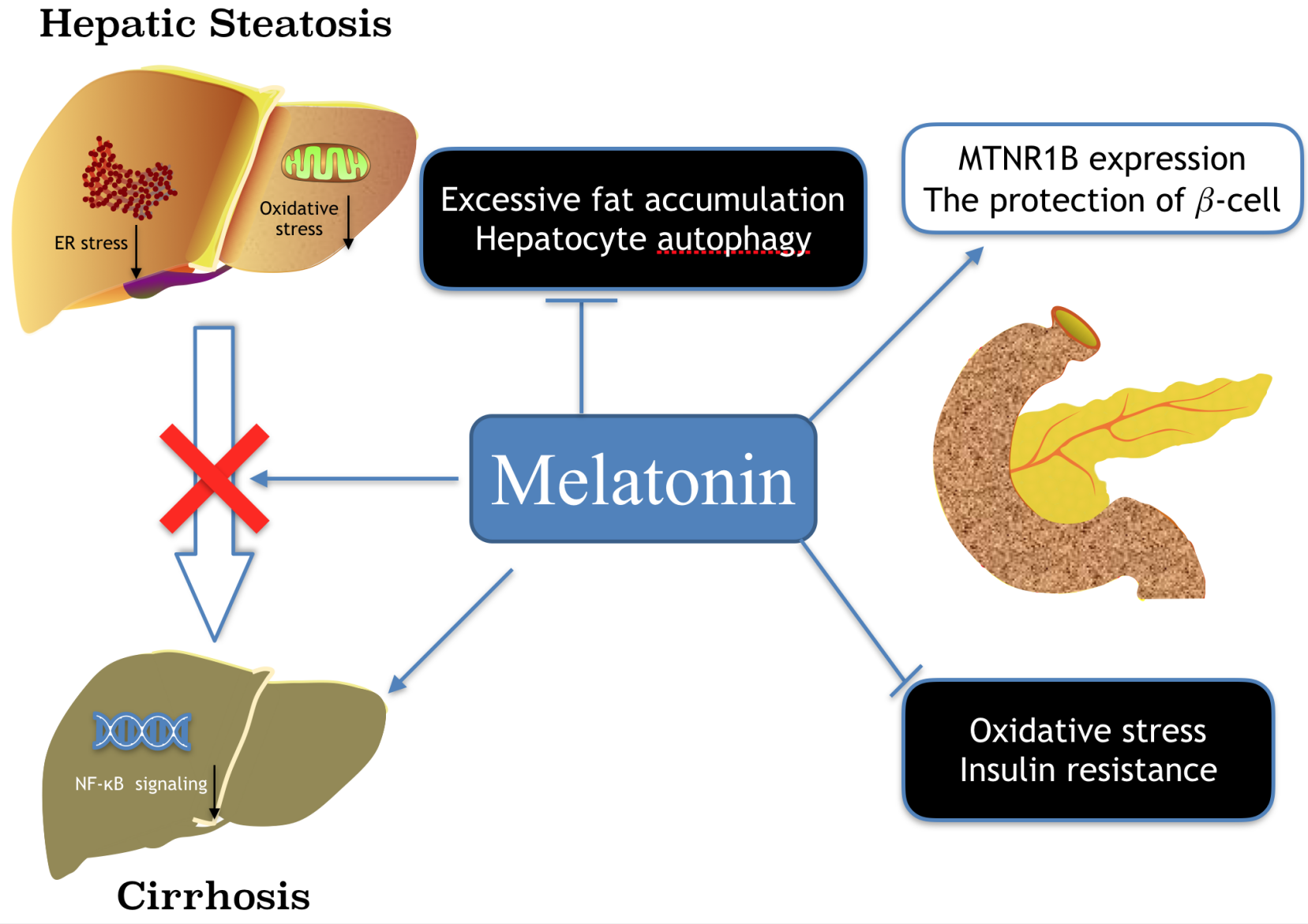
Melatonin is a well-known molecule for its involvement in circadian rhythm regulation and its contribution to protection against oxidative stress in organisms including unicellular alga, animals and plants. Currently, the bio-regulatory effects of melatonin on the physiology of various peripheral tissues have drawn a great attention of scientists. Although melatonin was previously defined as a neurohormone secreted from pineal gland, recently it has been identified that virtually, every cell has the capacity to synthesize melatonin and the locally generated melatonin has multiple pathophysiological functions, including regulations of obesity and metabolic syndromes. Herein, we focus on the effects of melatonin on fat deposition in various peripheral organs/tissues. The two important regulatory mechanisms related to the topic, i.e., the improvements of circadian rhythms and antioxidative capacity will be thoroughly discussed since they are linked to several biomarkers involved in obesity and energy imbalance, including metabolism and immunity. Furthermore, several other functions of melatonin which may serve to prevent or promote obesity and energy dysmetabolism-induced pathological states are also addressed. The organs of special interest include liver, pancreas, skeletal muscle, adipose tissue and the gut microbiota.
References
2. Spiegel K, Leproult R, Van Cauter E (1999) Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435-1439. doi: 10.1016/S0140-6736(99)01376-013768.
3. Turek FW, Joshu C, Kohsaka A, et al. (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043-1045. doi: 10.1126/science.1108750.
4. Liu Z, Gan L, Luo D, et al. (2017) Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J. Pineal Res. 62: doi: 10.1111/jpi.12383.
5. López A, García JA, Escames G, et al. (2009) Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 46: 188-198. doi: 10.1111/j.1600-079X.2008.00647.x.
6. Tan DX, Reiter R.J (2019) Mitochindria: the birthplace, battleground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-46. doi: 10.32794/mr11250011.
7. Gómez-Corvera A, Cerrillo I, Molinero P, et al. (2009) Evidence of immune system melatonin production by two pineal melatonin deficient mice, C57BL/6 and Swiss strains. J. Pineal Res. 47: 15-22. doi: 10.1111/j.1600-079X.2009.00683.x.
8. Chang MH, Wu UI, Lan CT (2009) Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep- deprived rats. J. Pineal Res. 47: 211-220. doi: 10.1111/j.1600-079X.2009.00704.x.
9. Jean-Louis G, von Gizycki H, Zizi F (1998) Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. J. Pineal Res. 25: 177-183. doi: 10.1111/j.1600-079x.1998.tb00557.x
10. Calvo JR, González-Yanes C, Maldonado MD (2013) The role of melatonin in the cells of the innate immunity: a review. J. Pineal Res. 55: 103‐120. doi: 10.1111/jpi.12075.
11. Mayo JC, Sainz RM, González Menéndez P, et al. (2017) Melatonin and sirtuins: A "not-so unexpected" relationship. J. Pineal Res. 62: e12391. doi: 10.1111/jpi.12391.
12. Alonso-Vale MI, Peres SB, Vernochet C, et al. (2009) Adipocyte differentiation is inhibited by melatonin through the regulation of C/EBPbeta transcriptional activity. J. Pineal Res. 47: 221-227. doi: 10.1111/j.1600-079X.2009.00705.x.
13. Tahan V, Atug O, Akin H, et al. (2009) Melatonin ameliorates methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in rats. J. Pineal Res. 46: 401-407. doi: 10.1111/j.1600-079X.2009.00676.x.
14. Shieh JM, Wu HT, Cheng KC, et al. (2009) Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKCzeta-Akt-GSK3beta pathway in hepatic cells. J. Pineal Res. 47: 339-344. doi: 10.1111/j.1600-079X.2009.00720.x.
15. Deng T, Lyon CJ, Bergin S, et al. (2016) Obesity, inflammation, and cancer. Ann. Rev. Pathol. 11: 421-449. doi: 10.1146/annurev-pathol-012615-044359.
16. Reiter RJ, Tan DX, Korkmaz A, et al. (2012) Obesity and metabolic syndrome: association with chronodisruption, sleep deprivation, and melatonin suppression. Ann. Med. 44: 564-577. doi: 10.3109/07853890.2011.586365.
17. Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15: 288-298. doi: 10.1038/s41574-019-0176-8.
18. Cipolla-Neto J, Amaral FG, Afeche SC, et al. (2014) Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56: 371-381. doi: 10.1111/jpi.12137.
19. Chen X, Zhang C, Zhao M, et al. (2011) Melatonin alleviates lipopolysaccharide-induced hepatic SREBP-1c activation and lipid accumulation in mice. J. Pineal Res. 51: 416-425. doi: 10.1111/j.1600-079X.2011.00905.x.
20. de Luxán-Delgado B, Caballero B, Potes Y, et al. (2014) Melatonin administration decreases adipogenesis in the liver of ob/ob mice through autophagy modulation. J. Pineal Res. 56: 126-133. doi: 10.1111/jpi.12104.
21. Sánchez DI, González-Fernández B, Crespo I, et al. (2018) Melatonin modulates dysregulated circadian clocks in mice with diethylnitrosamine-induced hepatocellular carcinoma. J. Pineal Res. 65: e12506. doi: 10.1111/jpi.12506.
22. Tan DX, Manchester LC, Fuentes-Broto L, et al. (2011) Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev. 12: 167-188. doi: 10.1111/j.1467-789X.2010.00756.x.
23. Rong B, Feng R, Liu C, et al. (2019) Reduced delivery of epididymal adipocyte-derived exosomal resistin is essential for melatonin ameliorating hepatic steatosis in mice. J. Pineal Res. 66: e12561. doi: 10.1111/jpi.12561.
24. Yin J, Li Y, Han H, et al. (2018) Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 65: e12524. doi: 10.1111/jpi.12524.
25. Fernández Vázquez G, Reiter RJ, Agil A (2018) Melatonin increases brown adipose tissue mass and function in Zücker diabetic fatty rats: implications for obesity control. J. Pineal Res. 64: e12472. doi: 10.1111/jpi.12472.
26. Han L, Wang H, Li L, et al. (2017) Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J. Pineal Res. 63: e12431. doi: 10.1111/jpi.12431.
27. Laothong U, Pinlaor P, Hiraku Y, et al. (2010) Protective effect of melatonin against Opisthorchis viverrini-induced oxidative and nitrosative DNA damage and liver injury in hamsters. J. Pineal Res. 49: 271-282. doi: 10.1111/j.1600-079X.2010.00792.x.
28. Tain YL, Hsieh CS, Chen CC, et al. (2010) Melatonin prevents increased asymmetric dimethylarginine in young rats with bile duct ligation. J. Pineal Res. 48: 212-221. doi: 10.1111/j.1600-079X.2010.00745.x.
29. de Luxán-Delgado B, Potes Y, Rubio-González A, et al. (2016) Melatonin reduces endoplasmic reticulum stress and autophagy in liver of leptin-deficient mice. J. Pineal Res. 61: 108-123.doi: 10.1111/jpi.12333.
30. Jung KH, Hong SW, Zheng HM, et al. (2009) Melatonin downregulates nuclear erythroid 2-related factor 2 and nuclear factor-kappaB during prevention of oxidative liver injury in a dimethylnitrosamine model. J. Pineal Res. 47: 173-183. doi: 10.1111/j.1600-079X.2009.00698.x.
31. Anstee QM, Reeves HL, Kotsiliti E, et al. (2019) From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16: 411-428. doi: 10.1038/s41575-019-0145-7.
32. Baiceanu A, Mesdom P, Lagouge M, et al. (2016) Endoplasmic reticulum proteostasis in hepatic steatosis. Nat. Rev. Endocrinol. 12:710-722. doi: 10.1038/nrendo.2016.124.
33. Ouyang X, Han SN, Zhang JY, et al. (2018) Digoxin suppresses pyruvate kinase M2-promoted HIF-1α transactivation in steatohepatitis. Cell Metab. 27: 339-350. doi: 10.1016/j.cmet.2018.04.007.
34. Koliaki C, Szendroedi J, Kaul K, et al. (2015) Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 21: 739-746. doi: 10.1016/j.cmet.2015.04.004.
35. Zaoualí MA, Reiter RJ, Padrissa-Altés S, et al. (2011) Melatonin protects steatotic and nonsteatotic liver grafts against cold ischemia and reperfusion injury. J. Pineal Res. 50: 213-221. doi: 10.1111/j.1600-079X.2010.00831.x.
36. Agil A, El-Hammadi M, Jiménez-Aranda A, et al. (2015) Melatonin reduces hepatic mitochondrial dysfunction in diabetic obese rats. J. Pineal Res. 59: 70-79. doi: 10.1111/jpi.12241.
37. Solís-Muñoz P, Solís-Herruzo JA, Fernández-Moreira D, et al. (2011) Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J. Pineal Res. 51: 113-123. doi: 10.1111/j.1600-079X.2011.00868.x.
38. Iñarrea P1, Casanova A, Alava MA, Iturralde M, Cadenas E (2011) Melatonin and steroid hormones activate intermembrane CU, ZN-superoxide dismutase by means of mitochondrial cytochrome P450. Free Radic. Biol. Med. 50: 1575-1581. doi: 10.1016/j.freeradbiomed.2011.03.003.
39. Tain YL, Kao YH, Hsieh CS, et al. (2010) Melatonin blocks oxidative stress-induced increased asymmetric dimethylarginine. Free Radic. Biol. Med. 49: 1088-1098. doi: 10.1016/j.freeradbiomed.2010.06.029.
40. Kim JY, Garcia-Carbonell R, Yamachika S, et al. (2018) ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell 175: 133-145. doi: 10.1016/j.cell.2018.08.020.
41. Heo JI, Yoon DW, Yu JH, et al. (2018) Melatonin improves insulin resistance and hepatic steatosis through attenuation of alpha-2-HS-glycoprotein. J. Pineal Res. 65: e12493. doi: 10.1016/j.cell.2018.08.020.
42. Zhou H, Du W, Li Y, et al. (2018) Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 64: e12450. doi: 10.1111/jpi.12450.
43. Das N, Mandala A, Naaz S, et al. (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J. Pineal Res. 62: e12404. doi: 10.1111/jpi.12404.
44. Hernandez-Gea V, Friedman SL (2011) Pathogenesis of liver fibrosis. Ann. Rev Pathol. 6: 425-456. doi: 10.1146/annurev-pathol-011110-130246.
45. Shajari S, Laliena A, Heegsma J, et al. (2015) Melatonin suppresses activation of hepatic stellate cells through RORα-mediated inhibition of 5-lipoxygenase. J. Pineal Res. 59: 391-401. doi: 10.1111/jpi.12271.
46. San-Miguel B, Crespo I, Sánchez DI, et al. (2015) Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride-induced fibrosis. J. Pineal Res. 59: 151-162. doi: 10.1111/jpi.12247.
47. Kitagawa A, Ohta Y, Ohashi K (2012) Melatonin improves metabolic syndrome induced by high fructose intake in rats. J. Pineal Res. 52: 403-413. doi: 10.1111/j.1600-079X.2011.00955.x.
48. Claustrat B, Brun J, Chazot G (2005) The basic physiology and pathophysiology of melatonin. Sleep MedRev. 9: 11-24. doi: 10.1016/j.smrv.2004.08.001.
49. Bonnefond A, Clément N, Fawcett K, et al. (2012) Rare MTNR1B variants impairing melatonin receptor1B function contribute to type 2 diabetes. Nat. Genet. 44: 297-301. doi: 10.1038/ng.1053.
50. von Gall C, Stehle JH, Weaver DR (2002) Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 309: 151-162. doi: 10.1007/s00441-002-0581-4.
51. Tuomi T, Nagorny CLF, Singh P, et al. (2016) Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 23: 1067-1077. doi: 10.1016/j.cmet.2016.04.009.
52. Lyssenko V, Nagorny CL, Erdos MR, et al. (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41: 82-88. doi: 10.1038/ng.288.
53. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, et al. (2009) A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 41: 89-94. doi: 10.1038/ng.277.
54. Rönn T, Wen J, Yang Z, et al. (2009) A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia 52: 830-833. doi: 10.1007/s00125-009-1297-8.
55. McMullan CJ, Schernhammer ES, Rimm EB, et al. (2013) Melatonin secretion and the incidence of type 2 diabetes. JAMA 309: 1388-1396. doi: 10.1001/jama.2013.2710.
56. Zanuto R, Siqueira-Filho MA, Caperuto LC, et al. (2013) Melatonin improves insulin sensitivity independently of weight loss in old obese rats. J. Pineal Res. 55: 156-165. doi: 10.1111/jpi.12056.
57. She M, Deng X, Guo Z, et al. (2009) NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol. Res. 59: 248-253. doi: 10.1016/j.phrs.2009.01.005.
58. Yoo YM (2013) Melatonin-mediated insulin synthesis during endoplasmic reticulum stress involves HuD expression in rat insulinoma INS-1E cells. J. Pineal Res. 55: 207-220. doi: 10.1111/jpi.12064.
59. Park JH, Shim HM, Na AY, et al. (2014) Melatonin prevents pancreatic β-cell loss due to glucotoxicity: the relationship between oxidative stress and endoplasmic reticulum stress. J. Pineal Res. 56: 143-153. doi: 10.1111/jpi.12106.
60. Cardinali DP, Vigo DE (2017) Melatonin, mitochondria, and the metabolic syndrome. Cell Mol. Life Sci. 74: 3941-3954. doi: 10.1007/s00018-017-2611-0.
61. Agil A, Reiter RJ, Jiménez-Aranda A, et al. (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 54: 381-388. doi: 10.1111/jpi.12012.
62. Agil A, Rosado I, Ruiz R, et al. (2012) Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J. Pineal Res. 52: 203-210. doi: 10.1111/j.1600-079X.2011.00928.x.
63. Agil A, Navarro-Alarcón M, Ruiz R, et al. (2011) Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J. Pineal Res. 50: 207-212. doi: 10.1111/j.1600-079X.2010.00830.x.
64. Jung KH, Hong SW, Zheng HM, et al. (2010) Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J. Pineal Res. 48: 239-250. doi: 10.1111/j.1600-079X.2010.00748.x.
65. Belyaev O, Herzog T, Munding J, et al. (2011) Protective role of endogenous melatonin in the early course of human acute pancreatitis. J. Pineal Res. 50: 71-77. doi: 10.1111/j.1600-079X.2010.00811.x.
66. Withyachumnarnkul B, Wongprapairot P, Trakulrungsi W (1989) Dynamic uptake of radioactive substance in rat salivary gland following 3H-melatonin administration. J. Pineal Res. 4: 169-175. doi: 10.1111/j.1600-079x.1987.tb00853.x
67. Lamosová D, Zeman M, Juráni M (1997) Influence of melatonin on chick skeletal muscle cell growth. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 118: 375-379. doi: 10.1016/s0742-8413(97)00159-x.
68. Ochoa JJ, Díaz-Castro J, Kajarabille N, et al. (2011) Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 51: 373-380. doi: 10.1111/j.1600-079X.2011.00899.x.
69. Borges Lda S, Dermargos A, da Silva Junior EP, et al. (2015) Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J. Pineal Res. 58: 166-172. doi: 10.1111/jpi.12202.
70. Bose G, Ghosh A, Chattopadhyay A, et al. (2019) Melatonin as a potential therapeutic molecule against myocardial damage caused by high fat diet (HFD). Melatonin Res. 2: 37-56. doi: 10.32794/mr11250030.
71. García-Prat L, Martínez-Vicente M, Perdiguero E, et al. (2016) Autophagy maintains stemness by preventing senescence. Nature 529: 37-42. doi: 10.1038/nature16187.
72. Teodoro BG, Baraldi FG, Sampaio IH, et al. (2014) Melatonin prevents mitochondrial dysfunction and insulin resistance in rat skeletal muscle. J. Pineal Res. 57: 155-167. doi: 10.1111/jpi.12157.
73. Kim CH, Kim KH, Yoo YM (2011) Melatonin protects against apoptotic and autophagic cell death in C2C12 murine myoblast cells. J. Pineal Res. 50: 241-249. doi: 10.1111/j.1600-079X.2010.00833.x.
74. Hong Y, Kim JH, Jin Y, et al. (2014) Melatonin treatment combined with treadmill exercise accelerates muscular adaptation through early inhibition of CHOP-mediated autophagy in the gastrocnemius of rats with intra-articular collagenase-induced knee laxity. J. Pineal Res. 56: 175-188. doi: 10.1111/jpi.12110.
75. Tezze C, Romanello V, Desbats MA, et al. (2017) Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 25: 1374-1389. doi: 10.1016/j.cmet.2017.04.021.
76. Bartelt A, Widenmaier SB, Schlein C, et al. (2018) Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 24: 292-303. doi: 10.1038/nm.4481.
77. Olescuck IF, Camargo LS, Carvalho PV, et al. (2019) Melatonin and brown adipose tissue: novel insights to a complex interplay. Melatonin Res. 2: 25-41. doi: 10.32794/mr11250039.
78. Bohan R, Tianyu X, Tiantian Z, et al. (2019) Gut microbiota: a potential manipulator for host adipose tissue and energy metabolism. J. Nutr. Biochem. 64: 206-217. doi: 10.1016/j.jnutbio.2018.10.020.
79. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. (2009) Cold activated brown adipose tissue in healthymen. N. Engl. J. Med .360: 1500-1508. doi: 10.1056/NEJMoa0808718.
80. Cypess AM, Lehman S, Williams G, et al. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl J. Med. 360: 1509-1517. doi: 10.1056/NEJMoa0810780.
81. Virtanen KA, Lidell ME, Orava J, et al. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009: 1518-1525. doi: 10.1056/NEJMoa0808949.
82. Kato H, Tanaka G, Masuda S, et al. (2015) Melatonin promotes adipogenesis and mitochondrial biogenesis in 3T3-L1 preadipocytes. J. Pineal Res. 59: 267-275. doi: 10.1111/jpi.12259.
83. Postes Y, Luxan-Delgado B, Rubio-Gonzales A, et al. (2019) Dose-dependent beneficial effect of melatonin on obesity: interaction of melatonin and leptin. Melatonin Res. 2: 1-8. doi:10.32794/mr11250008.
84. Liu Z, Gan L, Xu Y, et al. (2017) Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J. Pineal Res. 63: e12414. doi: 10.1111/jpi.12414.
85. Bahrami-Nejad Z, Zhao ML, Tholen S, et al. (2018) A transcriptional circuit filters oscillating circadian hormonal inputs to regulate fat cell differentiation. Cell Metab. 27: 854-868. doi: 10.1016/j.cmet.2018.03.012.
86. Chitraju C, Mejhert N, Haas JT, et al. (2017) Triglyceride synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Cell Metab. 26: 407-418. doi: 10.1016/j.cmet.2017.07.012.
87. Chung KJ, Chatzigeorgiou A, Economopoulou M, et al. (2017) A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat. Immunol. 18: 654-664. doi: 10.1038/ni.3728.
88. González A, Alvarez-García V, Martínez-Campa C, et al. (2012) Melatonin promotes differentiation of 3T3-L1 fibroblasts. J. Pineal Res. 52: 12-20. doi: 10.1111/j.1600-079X.2011.00911.x.
89. Marcheva B, Ramsey KM, Buhr ED, et al. (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466: 627-631. doi: 10.1038/nature09253.
90. Fu L, Patel MS, Bradley A, et al. (2005) The molecular clock mediates leptin-regulated bone formation. Cell 122: 803-815. doi: 10.1016/j.cell.2005.06.028.
91. Gaucher J, Montellier E, Sassone-Corsi P (2018) Molecular cogs: Interplay between circadian clock and cell cycle. Trends Cell Biol. 28: 368-379. doi: 10.1016/j.tcb.2018.01.006.
92. Bock FJ, Tait SWG (2020) Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 21 (2): 85-100. doi: 10.1038/s41580-019-0173-8.
93. Roberts AW, Davids MS, Pagel JM, et al. (2016) Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374: 311-322. doi: 10.1056/NEJMoa1513257.
94. Jimenéz-Aranda A, Fernández-Vázquez G, Mohammad A-Serrano M, et al. (2014) Melatonin improves mitochondrial function in inguinal white adipose tissue of Zücker diabetic fatty rats. J. Pineal Res. 57: 103-109. doi: 10.1111/jpi.12147.
95. Aglietti RA, Estevez A, Gupta A, et al. (2016) GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl. Acad. Sci. USA. 113: 7858-7863. doi: 10.1073/pnas.1607769113.
96. Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7: 99-109. doi: 10.1038/nrmicro2070.
97. Howrylak JA, Nakahira K (2017) Inflammasomes: key mediators of lung immunity. Ann. Rev. Physiol. 79: 471-494. doi: 10.1146/annurev-physiol-021115-105229.
98. Hammarstedt A, Gogg S, Hedjazifar S, et al. (2018) Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 98: 1911-1941. doi: 10.1152/physrev.00034.2017.
99. de Souza CAP, Gallo CC, de Camargo LS, et al. (2019) Melatonin multiple effects on brown adipose tissue molecular machinery. J. Pineal Res. 66: e12549. doi: 10.1111/jpi.12549.
100. Liu K, Yu W, Wei W, et al. (2019) Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J. Lipid Res. 60: 767-782. doi: 10.1194/jlr.M087619.
101. Jiménez-Aranda A, Fernández-Vázquez G, Campos D, et al. (2013) Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 55: 416-423. doi: 10.1111/jpi.12089.
102. Xu P, Wang J, Hong F, et al. (2017) Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 62: e12399.doi: 10.1111/jpi.12399.
103. Gao T, Wang Z, Dong Y, et al. (2019) Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 67: e12574. doi: 10.1111/jpi.12574.
104. Li G, Xie C, Lu S, et al. (2018) Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 26: 801. doi: 10.1016/j.cmet.2017.10.007.
105. Turnbaugh PJ, Ley RE, Mahowald MA, et al. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031.
106. Qin J, Li Y, Cai Z, et al. (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60. doi: 10.1038/nature11450.
107. Li T, Qi M, Gatesoupe FJ, et al. (2019) Adaptation to fasting in crucian carp (carassius auratus): gut microbiota and its correlative relationship with immune function. Microb. Ecol. 78: 6-19.
108. Catterson JH, Khericha M, Dyson MC, et al. (2018). Short-term, intermittent fasting induces long-lasting gut heath and tor-independent lifespan extension. Curr. Biol. 28: 1714–1724. doi: 10.1016/j.cub.2018.04.015.
109. Wei S, Zhao J, Wang S, et al. (2018) Intermittent administration of a leucine-deprived diet is able to intervene in type 2 diabetes in db/db mice. Heliyon 4: e00830. doi: 10.1016/j.heliyon.2018.e00830.
110. Manichanh C, Rigottier-Gois L, Bonnaud E, et al. (2018) Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55: 205-211. doi: 10.1136/gut.2005.073817.
111. Singh S, Dulai PS, Zarrinpar A, et al. (2017) Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 14: 110-121.doi: 10.1038/nrgastro.2016.181.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


