Melatonin supplementation protects against the benzo(e)pyrene cytotoxicity and optic cup formation disruption in chicken embryos
Melatonin counteracted benzo(e)pyrene embryotoxicity
Abstract
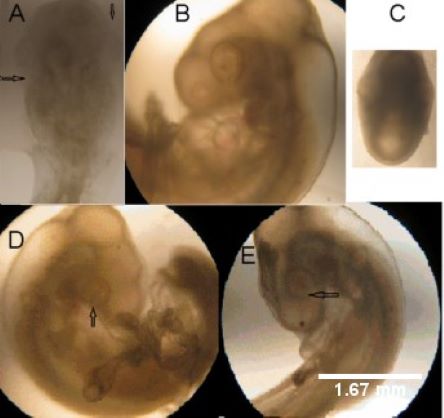
Benzo(e)pyrene is a cytotoxic chemical to the eyes, while neurohormone melatonin may exhibit protective effects on this cytotoxicity. In the current study, we have investigated the cytotoxic effects of benzo(e)pyrene on the chicken embryonic optic cups formation and whether melatonin supplementation protects chicken embryos against this xenobiotic toxicity. Fertilized chicken eggs were incubated for 48 h and then, they were divided into different groups. These groups included basal (without any treatment), control (distilled water), benzo(e)pyrene, melatonin and benzo(e)pyrene + melatonin groups, respectively. The 10 µl of distilled water or same volume of solution containing treatment compounds were injected into the air sac of the chicken egg. After an additional 18 h of incubation, the chicken embryos were excised and analyzed. The cytotoxicity was measured by a colorimetric whole chick embryo trypan blue assay. In embryos from basal, control and melatonin (0.01, 1 and 100 µM) groups, the frequency of the embryos with normal optic cups was 100% and had no increase in the embryonic cell death observed in post excision. In contrast, the frequency of normal optic cups in the benzo(e)pyrene (0.02 to 1200 µM) groups was significantly reduced (log IC50= -4.24 ± 0.02, R2= 0.98) with concentration-responsive manner. In addition, an increase in the embryonic cell death was also observed (log IC50 = -7.23 ± 0.28; R2 = 0.63). Melatonin treatment dose-responsively inhibited the benzo(e)pyrene-induced optic cups abnormality by 22.35 ± 4.06, 76.38 ± 3.30 and 100 % at the concentrations of 0.01, 1 and 100 µM, respectively. This same phenomenon was also observed in benzo(e)pyrene-induced embryonic cell death, i.e., melatonin suppressed the embryonic cell death by 16.67 ± 4.17, 54.17 ± 4.17 and 100 % with the abovementioned concentrations, respectively. Thus, melatonin supplementation injected into the chicken eggs protected against the benzo(e)pyrene embryotoxicity. Different pathways can be involved in melatonin’s protective effects.
References
2. Leon J, Acuña-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ (2004) Melatonin and mitochondrial function. Life Sci. 75: 765-790.
3. León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38: 1-9.
4. Acuña-Castroviejo D, Escames G, Rodriguez MI, Lopez LC (2007) Melatonin role in the mitochondrial function. Front. Biosci. 12: 947-963.
5. Acuña-Castroviejo D, López LC, Escames G, López A, García JA, Reiter RJ (2011) Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 11: 221-240.
6. Ganie SA, Dar TA, Bhat AH, Dar KB, Anees S, Zargar MA, Masood A (2016) Melatonin: A potential anti-oxidant therapeutic agent for mitochondrial dysfunctions and related disorders. Rejuvenation Res. 19: 21-40.
7. Hardeland R. Melatonin and the electron transport chain (2017) Cell Mol. Life Sci. 74: 3883-3896.
8. Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell. Mol. Life Sci. 74: 3863-3881.
9. Chang CC, Huang TY, Chen HY, Huang TC, Lin LC, Chang YJ, Hsia SM (2018) Protective effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid. Med. Cell Longev. 2018: 9015765.
10. Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G., Cecon E, Zlotos DP (2016) Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 173: 2702-2725.
11. Oblap R, Olszańska B (2001) Expression of melatonin receptor transcripts (mel-1a, mel-1b and mel-1c) in Japanese quail oocytes and eggs. Zygote 9: 237-244.
12. Oblap R, Olszańska B (2003) Presence and developmental regulation of serotonin Nacetyltransferase transcripts in oocytes and early quail embryos (Coturnix coturnix japonica). Mol. Reprod. Dev. 65: 132-140.
13. Olszańska B, Majewski P, Lewczuk B, Stepińska U (2007) Melatonin and its synthesizing enzymes (arylalkylamine N-acetyltransferase-like and hydroxyindole-O-methyltransferase) in avian eggs and early embryos. J. Pineal Res. 42: 310-318.
14. Sampaio RV, Conceição S, Miranda MS, Sampaio LF, Ohashi OM (2012) MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reprod. Biol. Endocrinol. 10:103.
15. Silva RN, Sampaio LFS (2014) Immunoreactivity of the Mel1a-like melatonin receptor and NRH: Quinone reductase enzyme (QR2) in testudines whole embryo and in developing whole retinas. Trends Dev. Biol. 8: 39-46.
16. Nogueira RC, Sampaio LFS (2017). Eye and heart morphogenesis are dependent on melatonin signaling in chick embryos. J. Exp. Biol. 220: 3826-3835.
17. Wu SP, Qian RR, Lee TC, Wang XH, Hong HS, Yuan CS (2012) Seasonal variation for the ratio of BaP to BeP at different sites in Great Xiamen Bay. J. Environ. Monit. 14: 1221-1230.
18. Fertmann R, Tesseraux I, Schümann M, Neus H (2002) Evaluation of ambient air concentrations of polycyclic aromatic hydrocarbons in Germany from 1990 to 1998. J Expo Anal. Environ. Epidemiol. 12: 115-123.
19. Johnson S, Persson Y, Frankki S, van Bavel B, Lundstedt S, Haglund P, Tysklind M (2007) Degradation of polycyclic aromatic hydrocarbons (PAHs) in contaminated soils by Fenton’s reagent: a multivariate evaluation of the importance of spoil characteristic and PAH properties. J. Hazard Mater. 149: 86-96.
20. Franci CD, Aleksieva A, Boulanger E, Brandenburg J, Johnston T, Malinova A, Head JA (2018) Potency of polycyclic aromatic hydrocarbons in chicken and Japanese quail embryos. Environ. Toxicol. Chem. 37: 1556-1564.
21. Revitt DM, Balogh T, Jones H (2014) Soil mobility of surface applied polyaromatic hydrocarbons in response to simulated rainfall. Environ. Sci. Pollut. Res. Int. 21: 4209-4219.
22. Shang J, Chen J, Shen Z, Xiao X, Yang H, Wang Y, Ruan A (2015) Photochemical degradation of PAHs in estuarine surface water: effects of DOM, salinity, and suspended particulate matter. Environ. Sci. Pollut. Res. Int. 22: 12374-12383.
23. Rodríguez-Acuña R, del Carmen Pérez-Camino M, Cert A, Moreda W (2008) Polycyclic aromatic hydrocarbons in spanish olive oils: relationship between benzo(a)pyrene and total polycyclic aromatic hydrocarbon content. J. Agric. Food Chem. 56: 10428-10432.
24. Kumosani TA, Moselhy SS, Asseri AM, Asseri AH (2013) Detection of polycyclic aromatic hydrocarbons in different types of processed foods. Toxicol. Ind. Health 29: 300-304.
25. Gelboin HV, Huberman E, Sachs L (1969) Enzymatic Hydroxylation of Benzopyrene and Its Relationship to Cytotoxicity. Proc. NatI. Acad. Sci. 64: 1188-1194.
26. Shimada T, Murayama N, Yamazaki H, Tanaka K, Takenaka S, Komori M, Kim D, Guengerich FP (2013) Metabolic activation of polycyclic aromatic hydrocarbons and aryl and heterocyclic amines by human cytochromes P450 2A13 and 2A6. Chem. Res. Toxicol. 26: 529-537.
27. Hamilton JW, Denison MS, Bloom SE (1983) Development of basal and induced aryl hydrocarbon (benzo[a]pyrene) hydroxylase activity in the chicken embryo in ovo. Proc. Natl. Acad. Sci U S A. 80: 3372-3376.
28. Estrago-Franco MF, Moustafa MT, Riazi-Esfahani M, Sapkal AU, Piche-Lopez R, Patil AJ, Sharma A, Falatoonzadeh P, Chwa M, Luczy-Bachman G, Kuppermann BD, Kenney MC (2016) Effects of benzo(e)pyrene on reactive oxygen/nitrogen species and inflammatory cytokines induction in human rpe cells and attenuation by mitochondrial-involved mechanism. J. Ophthalmic. Vis. Res. 11: 385-393.
29. Patil AJ, Gramajo AL, Sharma A, Chwa M, Seigel GM, Kuppermann BD, Kenney MC (2009) Effects of benzo(e)pyrene on the retinal neurosensory cells and human microvascular endothelial cells in vitro. Curr. Eye Res. 34: 672-682.
30. Sharma A, Neekhra A, Gramajo AL, Patil J, Chwa M, Kuppermann BD, Kenney MC (2008) Effects of Benzo(e)Pyrene, a toxic component of cigarette smoke, on human retinal pigment epithelial cells in vitro. Invest. Ophthalmol. Vis. Sci. 49: 5111-5117.
31. Mansoor S, Gupta N, Patil AJ, Estrago-Franco MF, Ramirez C, Migon R, Sapkal A, Kuppermann BD, Kenney MC (2010) Inhibition of apoptosis in human retinal pigment epithelial cells treated with benzo(e)pyrene, a toxic component of cigarette smoke. Invest. Ophthalmol. Vis. Sci. 51: 2601-2607.
32. Gupta NK, Mansoor S, Sapkal AU, Limb A, Kuppermann BD, MC, Kenney MC (2016) Effects of Benzo(e)Pyrene, a toxic component of cigarette smoke, on Müller Cells (MIO-M1) in vitro. New Front. Ophthalmol. 2: doi: 10.15761/NFO.1000124.
33. Man'cheva TA, Demidov DV, Plotnikova NA, Kharitonova TV, Pashkevich IV, Anisimov VN (2011) Melatonin and metformin inhibit skin carcinogenesis and lipid peroxidation induced by benz(a)pyrene in female mice. Bull. Exp. Biol. Med. 151: 363-365.
34. Miao Y, Zhou C, Bai Q, Cui Z, ShiYang X, Lu Y, Zhang M, Dai X, Xiong B (2018) The protective role of melatonin in porcine oocyte meiotic failure caused by the exposure to benzo(a)pyrene. Hum. Reprod. 33: 116-127.
35. Murawska-Ciałowicz E, Jethon Z, Magdalan J, Januszewska L, Podhorska-Okołów M, Zawadzki M, Sozański T, Dzięgiel P (2011) Effects of melatonin on lipid peroxidation and antioxidative enzyme activities in the liver, kidneys and brain of rats administered with benzo(a)pyrene. Exp. Toxicol. Pathol. 63: 97-103.
36. Vesnushkin GM, Plotnikova NA, Semenchenko AI, Anisimov VN (2006) Dose-dependent inhibitory effect of melatonin on carcinogenesis induced by benzo[a]pyrene in mice. J. Exp. Clin. Cancer Res. 25: 507-513.
37. Chang TK, Chen J, Yang G, Yeung EY (2010) Inhibition of procarcinogen-bioactivating human CYP1A1, CYP1A2 and CYP1B1 enzymes by melatonin. J. Pineal Res. 48: 55-64.
38. Sampaio LFS, Mesquita FP, de Sousa PR, Silva JL, Alves CN (2014) The melatonin analog 5-MCA-NAT increases endogenous dopamine levels by binding NRH: quinone reductase enzyme in the developing chick retina. Int. J. Dev. Neurosci. 38: 119-126.
39. Gesto M, Tintos A, Rodríguez-Illamola A, Soengas JL, Míguez JM (2009) Effects of naphthalene, beta-naphthoflavone and benzo(a)pyrene on the diurnal and nocturnal indoleamine metabolism and melatonin content in the pineal organ of rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 92:1-8.
40. Brady RC, Hilfer SR (1982) Optic cup formation: a calcium-regulated process. Proc. Natl. Acad. Sci. 79: 5587-5591.
41. Hamburger V, Hamilton, HL 1951 (1992) A series of normal stages in the development of the chick embryo. Dev. Dyn. 195: 231-272.
42. Hyer J, Kuhlman J, Afif E, Mikawa T (2003). Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Dev. Biol. 259: 351-363.
43. Fuhrmann S (2010) Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 93: 61-84.
44. Bruns RF, Menegatti CM, Martins WP, Araujo Júnior E (2015) Applicability of pocket ultrasound during the first trimester of pregnancy. Med. Ultrason. 17: 284-288.
45. Huska J, Myers B, Albanese S, Shenberger S. The Effect of Exposure to Ethanol onTranscriptional Levels of Sonic Hedgehog in 2-Day Old Chicks. http://sites.millersville.edu/jcebrathomas/cebra_thomas/DB_lab/Chick/Ethanol%20Exposure%20Decreases%20SHH.htm, assessed in September, 02, 2016.
46. Uliasz TF, Hewett SJ (2000) A microtiter trypan blue absorbance assay for the quantitative determination of excitotoxic neuronal injury in cell culture. J. Neurosci. Methods. 100: 157-163.
47. Liesche J, Marek M, Günther-Pomorski T (2015) Cell wall staining with Trypan blue enables quantitative analysis of morphological changes in yeast cells. Front. Microbiol. 6: 107.
48. Varghese F, Bukhari AB, Malhotra R, De A (2014) IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PloS one 9 (5): e96801.
49. Wood AW, Levin W, Thakker DR, Yagi H, Chang RL, Ryan DE, Thomas PE, Dansette PM, Whittaker N, Turujman S, Lehr RE, Kumar S, Jerina DM, Conney AH (1979) Biological activity of benzo[e]pyrene. An assessment based on mutagenic activities and metabolic profiles of the polycyclic hydrocarbon and its derivatives. J. Biol. Chem. 254: 4408-4415.
50. Jolles B, Demerseman P, Chalvet O, Strapelias H, Herning T, Royer R, Duquesne M (1987) Reactivity with DNA of three pyrenofuran analogues of benzo(a)pyrene and benzo(e)pyrene. Nucleic Acids Res. 15: 9487-9497.
51. Houser WH, Raha A, Vickers M (1992) Induction of CYP1A1 gene expression in H4-II-E rat hepatoma cells by benzo[e]pyrene. Mol. Carcinog. 5: 232-237.
52. Lau HH, Baird WM (1992) The co-carcinogen benzo[e]pyrene increases the binding of a low dose of the carcinogen benzo[a]pyrene to DNA in Sencar mouse epidermis. Cancer Lett. 63: 229-236.
53. Hoffman DJ, Gay ML (1981) Embryotoxic effects of benzo[a]pyrene, chrysene, and 7,12-dimethylbenz[a]anthracene in petroleum hydrocarbon mixtures in mallard ducks. J. Toxicol. Environ. Health 7: 775-787.
54. Anwer J, Mehrotra NK. Effect of simultaneous exposure to nickel chloride and benzo(a)pyrene on developing chick embryos (1986) Drug Chem. Toxicol. 9: 171-183.
55. Stoncius D, Lazutka JR (2003) Spontaneous and benzo[a]pyrene-induced micronuclei in the embryos of the black-headed gull (Larus ridibundus L.). Mutat. Res. 538: 31-39.
56. Krieger JA, Born JL, Burchiel SW (1994) Persistence of calcium elevation in the HPB-ALL human T cell line correlates with immunosuppressive properties of polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 127: 268-274.
57. Krieger JA, Davila DR, Lytton J, Born JL, Burchiel SW (1995) Inhibition of sarcoplasmic/endoplasmic reticulum calcium ATPases (SERCA) by polycyclic aromatic hydrocarbons in HPB-ALL human T cells and other tissues. Toxicol. Appl. Pharmacol. 133: 102-108.
58. Mayati A, Le Ferrec E, Lagadic-Gossmann D, Fardel O (2012) Aryl hydrocarbon receptor-independent up-regulation of intracellular calcium concentration by environmental polycyclic aromatic hydrocarbons in human endothelial HMEC-1 cells. Environ. Toxicol. 27: 556-562.
59. Ji X, Chou X, Ge Z, Ding F, Gao H, Wu Q (2018) Benzo[a]pyrene-decreased gap junctional intercellular communication via calcium/calmodulin signaling increases apoptosis in TM4 cells. J. Appl. Toxicol. 38: 1091‐1103.
60. Humeau J, Bravo-San Pedro JM, Vitale I, Nuñez L, Villalobos C, Kroemer G, Senovilla L (2018) Calcium signaling and cell cycle: Progression or death. Cell Calcium 70: 3-15.
61. Kamiya T, Nagaoka T, Omae T, Ono S, Otani S, Yoshida A (2017) Benzo(e)pyrene Inhibits endothelium-dependent no-mediated dilation of retinal arterioles via superoxide production and endoplasmic reticulum stress. Invest. Ophthalmol. Vis. Sci. 58: 5978-5984.
62. Chen F, Reheman A, Cao J, Wang Z, Dong Y, Zang Y, Chen Y (2016) Effect of melatonin on monochromatic light-induced T-lymphocyte proliferation in the thymus of chickens. J. Photochem. Photobiol. B. 161: 9‐16.
63. Pegan SD, Sturdy M, Ferry G, Delagrange P, Boutin JA, Mesecar AD (2011) X-ray structural studies of quinone reductase 2 nanomolar range inhibitors. Protein Sci. 20: 1182‐1195.
64. Navarro Gil FJ, Huete-Toral F, Crooke A, Dominguez Godinez CO, Carracedo G, Pintor J (2019) Effect of melatonin and its analogs on tear secretion. J. Pharmacol. Exp. Ther. 371: 186‐190.
65. Shimada T, Inoue K, Suzuki Y, Kawai T, Azuma E, Nakajima T, Shindo M, Kurose K, Sugie A, Yamagishi Y, Fujii-Kuriyama Y, Hashimoto M (2002) Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. Carcinogenesis 23: 1199-1207.
66. Stejskalova L, Dvorak Z, Pavek P (2011) Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr. Drug Metab. 12:198-212.
67. Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP (2000) Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol. 59: 65-85.
68. Kawajiri K, Fujii-Kuriyama Y (2017) The aryl hydrocarbon receptor: a multifunctional chemical sensor for host defense and homeostatic maintenance. Exp. Anim. 66: 75-89.
69. Gibbons JA, Babish JG (1992) Benzo[e]pyrene elicits changes in the biochemical activities and chromatographic behavior of murine hepatic cytochromes P-450 that are distinct from those induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem. Biol. Interact. 83: 203-220.
70. Curtis LR, Garzon CB, Arkoosh M, Collier T, Myers MS, Buzitis J, Hahn ME (2011) Reduced cytochrome P4501A activity and recovery from oxidative stress during subchronic benzo[a]pyrene and benzo[e]pyrene treatment of rainbow trout. Toxicol. Appl. Pharmacol. 254:1-7.
71. Sterling K, Raha A, Bresnick E (1994) Induction of CYP1A1 gene expression in mouse hepatoma cells by benzo[e]pyrene, a ligand of the 4S polycyclic hydrocarbon-binding protein. Toxicol. Appl. Pharmacol. 128:18-24.
72. Raha A, Hamilton JW, Bresnick E (1999) The existence of the 4S polycyclic aromatic hydrocarbon-protein binding in 14-day-old chick embryo liver. Toxicol. Appl. Pharmacol. 158:1-8.
73. Earnshaw WC, Martins LM, Kaufmann SH (1990) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68: 383-424.
74. Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 65(1): e12514.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


