Melatonin as a potential therapeutic molecule against COVID-19 associated gastrointestinal complications: An unrevealed link
COVID-19 and gastrointestinal melatonin
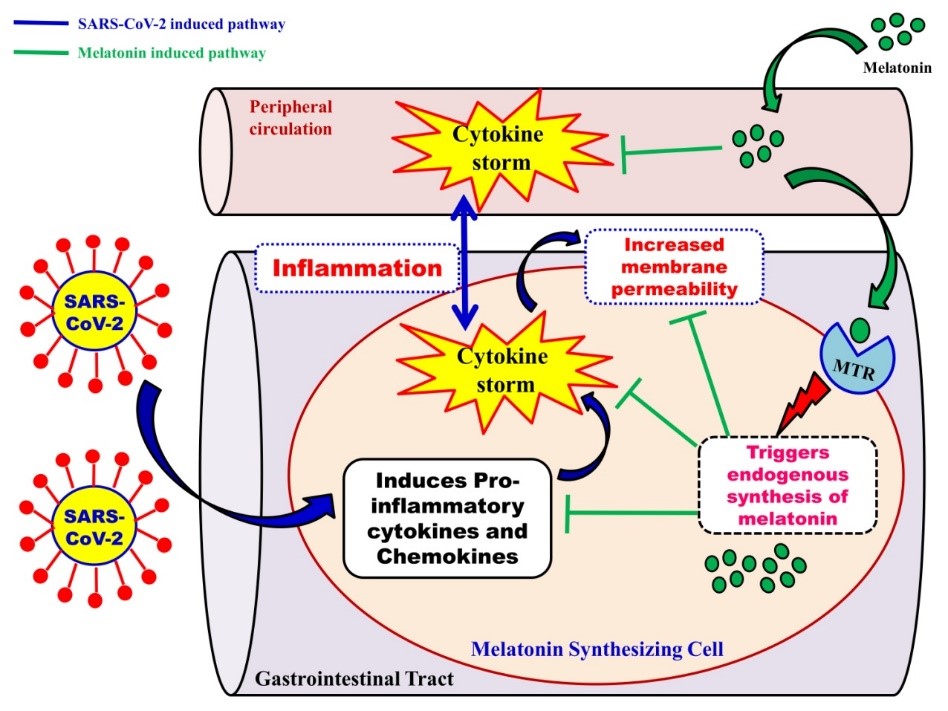
Abstract
Outbreak of the novel coronavirus disease (COVID-19) was first reported in Wuhan, Hubei province of China, in early December 2019 which was later declared as a pandemic by World Health Organization (WHO) in March 2020. The International Committee on Taxonomy of Viruses has termed this novel coronavirus as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). According to the report of WHO on 29th April, 2020, 3018681 confirmed cases along with 207973 deaths have been documented globally. COVID-19 was originally reported as a lethal lung disease with fever and cough as the most common symptoms; however, the increasing number of gastrointestinal symptoms, such as diarrhoea, vomiting and abdominal pain in patients have clearly suggested that gastrointestinal tract (GIT) may also serve as a potential route for SARS-CoV-2 infection. To identify the effective therapies on this pandemic is urgent. Keeping this in mind, we realize that melatonin is a potent antioxidant, anti-inflammatory and immunomodulatory molecule and it has been used in diverse diseases and pathophysiological conditions, including respiratory disease and viral infections. Importantly, melatonin specific receptors and its endogenously synthetic machinery are distributed throughout the mammalian gastrointestinal system. Therefore, the therapeutic potentiality of melatonin in SARS-CoV-2 associated digestive symptoms cannot be ignored. In this review, we focus on the clinical implications of melatonin on the digestive complications associated with SARS-CoV-2 infection.
References
2. Gu J, Han B, Wang J (2020) COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology S0016-5085(20)30281-X.
3. Ng SC, Tilg H (2020) COVID-19 and the gastrointestinal tract: more than meets the eye. Gut. pii: gutjnl-2020-321195. DOI: 10.1136/gutjnl-2020-321195.
4. Phelan AL, Katz R, Gostin LO (2020) The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA DOI: 10.1001/jama.2020.1097.
5. Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, Kuang L, Fang X, Mishra N, Lu J, Shan H, Jiang G, Huang X (2020) Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 5: 434-435. DOI: 10.1016/S2468-1253(20)30083-2.
6. Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra JC, Stöhr K, Peiris JS, Osterhaus AD (2003) Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362: 263-270. DOI: 10.1016/S0140-6736(03)13967-0.
7. de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LL, Snijder EJ, Stephens GM, Woo PC, Zaki AM, Zambon M, Ziebuhr J (2013) Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J. Virol .87: 7790-7792. DOI: 10.1128/JVI.01244-13.
8. Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF (2016) Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 24: 490-502. DOI: 10.1016/j.tim.2016.03.003.
9. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet 395: 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
10. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507-513. DOI: 10.1016/S0140-6736(20)30211-7.
11. Guan W, Ni Z, Hu Y, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382: 1708-1720. DOI: 10.1056/NEJMoa2002032.
12. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506. DOI: 10.1016/S0140-6736(20)30183-5.
13. Leung JM, Yang CX, Tam Anthony, Shaipanich T, Hackett TL, Singhera GK, Dorscheid DR, Sin DD (2020) ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications of COVID-19. Eur. Respir. J. DOI: 10.1183/13993003. 00688-2020.
14. Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Li Z, Cui X, Xiao J, Zhan J, Meng T, Zhou W, Liu J, Xu H (2020) Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 0: 1–9. DOI: 10.1136/gutjnl-2020-320953.
15. Huang SH, Cao XJ, Liu W, Shi XY, Wei W (2010) Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. 48: 109–116. https://doi.org/10.1111/j.1600-079X.2009.00733.x.
16. Yip HK, Chang YC, Wallace CG, Chang LT, Tsai TH, Chen YL, Chang HW, Leu S, Zhen YY, Tsai CY, Yeh KH, Sun CK, Yen CH (2013) Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J. Pineal Res. 54: 207–221. https://doi.org/10.1111/jpi.12020.
17. Wu X, Ji H, Wang Y, Gu C, Gu W, Hu L, Zhu L (2019) Melatonin alleviates radiation- induced lung injury via regulation of miR-30e/NLRP3 axis, Oxidative Med. Cell. Longev. 2019: 4087298. https://doi.org/10.1155/2019/4087298.
18. Reiter RJ, Ma Q, Sharma R (2020) Treatment of Ebola and other infectious diseases: melatonin “goes viral”. Melatonin Res. 3: 43–57. https://doi.org/10.32794/ mr11250047.
19. Bubenik GA (1999) Localization and physiological significance of gastrointestinal melatonin. In: R Watson (ed.) Melatonin in health promotion. CRC press, Boca Raton, Florida, pp. 21–39.
20. Kvetnoy IM, Ingel IE, Kvetnaia TV Malinovskaya NK, Rapoport SI, Raikhlin NT, Trofimov AV, Yuzhakov VV (2002) Gastrointestinal melatonin: cellular identification and biological role. Neuro. Endocrinol. Lett. 23: 121–132.
21. Pal PK, Bhattacharjee B, Ghosh A, Chattopadhyay A, Bandyopadhyay D (2018) Adrenaline induced disruption of endogenous melatonergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Res.1: 109–131. DOI: 10.32794/mr11250007.
22. Pal PK, Sarkar S, Chattopadhyay A, Tan DX, Bandyopadhyay D (2019) Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals. Melatonin Res. 2 (4): 61-82. DOI: https://doi.org/https://doi.org/10.32794 /mr11250041.
23. Hu LL, Wang WJ, Zhu QJ, Yang L (2020) Novel coronavirus pneumonia related liver injury: etiological analysis and treatment strategy. Zhonghua Gan Zang Bing Za Zhi. 28: E001.
24. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. DOI: 10.1016/S2213-2600(20)30079-5.
25. Yao N, Wang SN, Lian JQ, Sun YT, Zhang GF, Kang WZ, Kang W (2020) Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region. Zhonghua Gan Zang Bing Za Zhi. 28: E003. DOI: 10.3760/cma.j.cn501113-20200226-00070.
26. Perlman S, Netland J (2009) Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol.7: 439-450.
27. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, et al., (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395 (10224): 565-574. DOI: 10.1016/S0140-6736(20)30251-8.
28. Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, Wu KC, Chen MH (2020) Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet 5: 426-428. https://doi.org/10.1016/S2468-1253(20)30076-5.
29. Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, et al., (2017) A single-cell survey of the small intestinal epithelium. Nature 551 (7680): 333-339. DOI: 10.1038/nature24489.
30. Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O'Ryan M, Kang G, Desselberger U, Estes MK (2017) Rotavirus infection. Nat. Rev. Dis. Primers. 3: 17083. DOI: 10.1038/nrdp.2017.83.
31. Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, et al. (2016) Replication of human noroviruses in stem cell-derived human enteroids. Science 353: 1387–1393. DOI: 10.1126/science.aaf5211.
32. Desmarets LMB, Theuns S, Roukaerts IDM, Acar DD, Nauwynck HJ (2014) Role of sialic acids in feline enteric coronavirus infections. J. Gen. Virol. 95: 1911–1918. DOI: 10.1099/vir.0.064717-0.
33. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. (2020) First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382: 929–936. DOI: 10.1056/NEJMoa2001191.
34. Tang A, Tong ZD, Wang HL, Dai YX, Li KF, Liu JN, Wu WJ, Yuan C, Yu ML, Li P, Yan JB (2020) Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis.26 (6): DOI: 10.3201/eid2606.200301.
35. Xie C, Jiang L, Huang G, Pu H, Gong B, Lin H et al. (2020) Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 93: 264-267. DOI: 10.1016/j.ijid.2020.02.050.
36. Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu Lei (2020) Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. DOI: 10.14309/ajg.0000000000000620.
37. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. DOI: 10.1001/jama.2020.
38. Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM (2017) Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 15: 55-63.
39. He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J (2017) Gut-lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 43: 81-95.
40. Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, Carrara S, Fugazza A, DiLeo M, Galtieri PA, Pellegatta G, Ferrara EC, Azzolini E, Lagioia M (2020) Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest. Endosc. 1-6. https://doi.org/10.1016/j.gie.2020.03.019.
41. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al., (2020) Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv DOI: https://doi.org/10.1101/2020.02.03.931766.
42. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol.21 (3): 335-337. DOI: 10.1016/S1470-2045(20)30096-6.
43. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al., (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. DOI: 10.1038/s41586-020-2012-7.
44. Powers JH, Bacci ED, Guerrero ML, Leidy NK, Stringer S, Kim K, et al., (2018) Reliability, validity, and responsiveness of influenza patient-reported outcome (FLU-PRO©) scores in influenza-positive patients. Value Health. 21: 210–218. DOI: 10.1016/j.jval.2017.04.014.
45. Gui M, Song W, Zhou H, Xu J, Chen S, Xiang Y, Wang X (2017) Cryo- electron microscopy structures of the SARS–coV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 27: 119–129. https://doi.org/10.1038/cr.2016.152.
46. Li et al., 2005 Li F, Li W, Farzan M (2005) Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309: 1864–1868.
47. Song W, Gui M, Wang X, Xiang Y (2018) Cryo- em structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 14: e1007236. DOI: 10.1371/journal.ppat.1007236.
48. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263. DOI: 10.1126/science.abb2507.
49. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P (2020) Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life. Sci. 63: 457–460. DOI: 10.1007/s11427-020-1637-5.
50. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al., (2020) SARS-coV-2 cell entry depends on ACE2 and TMPRSS 2 and is blocked by a clinically proven protease inhibitor. Cell 181 (2): 271-280.e8.DOI: 10.1016/j.cell.2020.02.052.
51. Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S (2014) Tmprss2 and ADAM17 cleave ace2 differentially and only proteolysis by TMPRSS 2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 88: 1293–1307. DOI: 10.1128/JVI.02202-13.
52. Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R Jr, Nunneley JW, et al. (2015) Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 116: 76–84. DOI: 10.1016/j.antiviral.2015.01.011.
53. Meng T, Cao H, Zhang H, Kang Z, Xu D, Gonh H, et al., (2020) The insert sequence in SARS- coV-2 enhances spike protein cleavage by TMPRSS. bioRxiv DOI: https://doi.org/10.1101/2020.02.08.926006.
54. Cheung CY, Poon LLM, Ng IHY, Luk W, Sia SF, Wu MHS, Chan KH, Yuen Y, Gordon S, Guan Y, Peiris JSM (2005) Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 797819–797826. https://doi.org/10.1128/JVI.79.12.7819-7826.2005.
55. Law et al., 2005 Law HKW, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Nicholls JM, Peiris JSM, Lau YL (2005) Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 106: 2366–2374. https://doi.org/ 10.1182/ blood-2004-10-4166.
56. Chu et al., 2016 Chu H, Zhou J, Wong BHY, Li CC, Chan JFW, Cheng ZS, Yang D, Wang D, Lee ACY, Li CC, Yeung ML, Cai JP, Chan IHY, Ho WK, To KKW, Zheng BJ, Yao Y, Qin C, Yeun KY (2016) Middle east respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 213: 904–914. https://doi.org/10.1093/infdis/jiv380.
57. Byrnes JJ, Gross S, Ellard C, Connolly K, Donahue S, Picarella D (2009) Effects of the ACE2 inhibitor GL1001 on acute dextran sodium sulfate-induced colitis in mice. Inflamm. Res. 58: 819-827. https://doi.org/10.1007/s00011-009-0053-3.
58. Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK (2008) Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 143: 334-342.
59. Hardeland R (2018) Review Melatonin and retinoid orphan receptors: Demand for new interpretations after their exclusion as nuclear melatonin receptors. Melatonin Res.1: 78-93. DOI: 10.32794/mr11250005.
60. Kucukakin B, Lykkesfeldt J, Nielsen HJ, Reiter RJ, Rosenberg J, Gogenur I (2008) Utility of melatonin to treat surgical stress after major vascular surgery–a safety study. J. Pineal Res. 44: 426–431. https://doi.org/10.1111/j.1600-079X.2007.00545.x.
61. Zhao Z, Lu C, Li T, Wang W, Ye W, Zeng R, Ni L, Lai Z, Wang X, Liu C (2018) The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: in vivo assessment and a randomized controlled trial. J. Pineal Res. 65: e12521, https://doi.org/10.1111/jpi.12521.
62. Shafiei E, Bahtoei M, Raj P, Ostovar A, Iranpour D, Akbarzadeh S, Shahryari H, Anvaripour A, Tahmasebi R, Netticadan T, Movahed A (2018) Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: a randomized, open-labeled, placebo-controlled trial. Medicine 97: e11383, https://doi.org/10.1097/MD.0000000000011383.
63. Huo X, Wang C, Yu Z, Peng Y, Wang S, Feng S, Zhang S, Tian X, Sun C, Liu K, Deng S, Ma X (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 62 (4): e12390. DOI: 10.1111/jpi.12390.
64. Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234‐250. DOI:10.1111/jpi.12210.
65. Pal PK, Maitra SK (2018) Response of gastrointestinal melatonin, antioxidants, and digestive enzymes to altered feeding conditions in carp (Catla catla). Fish Physiol. Biochem. 44: 1061–1073. https://doi.org/10.1007/s10695-018-0494-0.
66. Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX (2000) Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 917: 376–386. https://doi.org/10.1111/j.1749-6632.2000.tb05402.x.
67. Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36: 1–9.
68. Cuzzocrea S, Reiter RJ (2001) Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur. J. Pharmacol. 426: 1–10. https://doi.org/10.1016/S0014-2999(01)01175-X.
69. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J (2013) A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J. Pineal Res. 54: 1–14.doi: 10.1111/j.1600-079X.2012.01014.x.
70. Mitra E, Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Res.2: 1-21. DOI: 10.32794/mr11250018.
71. Tan DX, Hardeland R (2020) Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: focus on COVID-19. Melatonin Res. 3: 120-143. DOI: 10.32794/mr11250052.
72. Ben-Nathan D, Maestroni GJ, Lustig S, Conti A (1995) Protective effects of melatonin in mice infected with encephalitis viruses. Arch. Virol. 140: 223–230.
73. Raikhlin NT, Kvetnoy IM (1974) Lightening effect of the extract of human appendix mucosa on frog skin melanophores. Bull. Exp. Biol. Med. 8: 114–116.
74. Fu Z, Kato H, Kotera N, Noguchi T, Sugahara K, Kubo T (2001) Regulation of hydroxyindole-O-methyltransferase gene expression in Japanese quail (Coturnixcoturnix japonica). Biosci. Biotechnol. Biochem. 65: 2504–2511. DOI: 10.1271/bbb.65.2504.
75. Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R (2008) Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab. 19: 17–24.
76. Hong GX, Pang SF (1995) N-Acetyltransferase activity in the quail (Coturnixcoturnix japonica) duodenum. Comp. Biochem. Physiol. 112: 251–255.
77. Dobocovich M, Mankowsha M (2005) Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27: 101–110. DOI: 10.1385/ENDO:27:2:101.
78. Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Bielanski W, Brzozowska I, Stachura J, Han EG (1997) The role of melatonin and L-tryptophan in prevention of acute gastric lesions induced by stress, ethanol, ischemia, and aspirin. J. Pineal Res. 23: 79-89. https://doi.org/10.1111/j.1600-079X.1997.tb00339.x.
79. Witt-Enderby PA, Radio NM, Doctor JS, Davis VL (2006) Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 41: 297–305. DOI: 10.1111/j.1600-079x.2006.00369.x.
80. Srinivasan V, Lauterbach EC, Ho KY, Acuña-Castroviejo D, Zakaria R, Brzezinski A (2012) Melatonin in antinociception: Its therapeutic applications. Curr. Neuropharmacol. 10: 167–178. DOI: 10.2174/157015912800604489.
81. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181-97. https://www.ncbi.nlm.nih.gov/pubmed /11899100.
82. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253–278. DOI: 10.1111/jpi.12360.
83. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66. DOI: https://doi.org/10.32794/ mr11250011.
84. Reiter RJ, Tan DX, Manchester LC, Qi W. (2001) Biochemical reactivity of melatonin with reactive oxygen and reactive nitrogen species: A review of the evidence. Cell Biochem. Biophys. 34: 237–256. DOI: 10.1385/CBB:34:2:237.
85. Cuzzocrea S, Reiter JR (2002) Pharmacological action of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2: 153–165.
86. Nosál'ová et al., 2007 Nosál'ová V, Zeman M, Černá S, Navarová J, Zakálová M (2007) Protective effect of melatonin in acetic acid induced colitis in rats. J. Pineal Res. 42: 364–370. DOI: 10.1111/j.1600-079X.2007.00428.x.
87. Torres-Farfan C, Richter HG, Rojas-García P, Vergara M, Forcelledo ML, Valladares LE, Torrealba F, Valenzuela GJ, Seron- Ferre M. (2003) mt1 Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 88: 450–458. DOI: 10.1210/jc.2002-021048.
88. Saito S, Tachibana T, Choi YH, Denbow DM, Furuse M (2005) ICV melatonin reduces stress responses in neonatal chicks. Behav. Brain Res. 165: 197–203. DOI: 10.1016/j.bbr.2005.06.045.
89. Lopez-Burillo S, Tan DX, Rodriguez-Gallego V, Manchester LC, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin and its derivatives cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5- methoxykynuramine, and 6-hydroxymelatonin reduced oxidative damage induced by Fenton reagents. J. Pineal Res. 34: 237–256. DOI: 10.1034/j.1600-079x.2003.00025.x.
90. Imai Y, Kuba K, Neely G, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YHC, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JSM, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM (2008) Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–249, https://doi.org/10.1016/j.cell.2008.02.043.
91. Mosca A, Leclerc M, Hugot JP (2016) Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front. Microbiol. 7: 1–12. https://doi.org/10.3389/fmicb.2016.00455.
92. Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, Che T, Zhang C (2019) Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 8 (3): 1–28. https://doi.org/10.3390/ pathogens 8030126.
93. Bingula R, Filaire M, Radosevic-Robin N, Bey M, Berthon JY, Bernalier-DonadilleA, Vasson MP, Filaire E (2017) Desired turbulence? Gut-lung Axis, immunity, and lung Cancer. J. Oncol. 2017: 5035371. https://doi.org/10.1155/2017/5035371.
94. Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y (2020) The cross-talk between gut microbiota and lungs in common lung diseases. Front. Microbiol. 11: 1–14. https://doi.org/10.3389/fmicb. 2020.00301.
95. Keely S, Talley NJ, Hansbro PM (2012) Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 5 (1): 7–18. https://doi.org/10.1038/mi.2011.55.
96. Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O (2018) The role of the lung microbiota and the gut–lung axis in respiratory infectious diseases. Cell. Microbiol.20(12): e12966. https://doi.org/10.1111/cmi.12966.
97. Dickson RP, Arbor A (2017) The microbiome and critical illness. Lancet Respir. Med. 4 (1): 59–72. https://doi.org/10.1016/S2213-2600(15)00427-0.
98. Negi S, Das DK, Pahari S, Nadeem S, Agrewala JN (2019) Potential role of gut microbiota in induction and regulation of innate immune memory. Front. Immunol. 10: 1–12. https://doi.org/10.3389/fimmu.2019.02441.
99. Round JL, Mazmanian SK (2010) Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. 107 (27): 12204–12209. https://doi.org/10.1073/pnas.0909122107.
100. Domínguez-Díaz C, García-Orozco A, Riera-Leal A, Padilla-Arellano JR, Fafutis-Morris M (2019) Microbiota and its role on viral evasion: Is it with us or against us? Front. Cell. Infect. Microbiol. 9: 256. https://doi.org/10.3389/fcimb.2019.00256.
101. Dhara D, Mohanty A (2020) Gut microbiota and Covid-19- possible link and implications. Virus Res. 285: 198018. https://doi.org/10.1016/j.virusres.2020.198018.
102. Groves HT, Higham SL, Moffatt MF, Cox MJ, Tregoning JS (2020) Respiratory viral infection alters the gut microbiota by inducing inappetence. mBio. 11: e03236-19. https://doi.org/10.1128/mBio.03236-19.
103. Zuo T, Zhang F, Lin GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung A, Cheung CP, Chen N, Lai CKC, Chen Z, Fung ESC, Chan V, Ling L, Joynt G, Hui DSC, Ng AC (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology https://doi.org/10.1053/j.gastro.2020.05.048.
104. Hajifathalian K, Mahadev S, Schwartz RE, Shah S, Sampath K, Schnoll-Sussman F, Brown Jr RS, Carr-Locke D, Cohen DE, Sharaiha RZ (2020) SARS-COV-2 infection (coronavirus disease 2019) for the gastrointestinal consultant. World J. Gastroenterol. 26 (14): 1546–1553. DOI: 10.3748/wjg.v26.i14.1546.
105. Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H (2020) Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 69 (6): 997‐1001. DOI: 10.1136/gutjnl-2020-321013.
106. Paulose JK, Cassone VM (2016) The melatonin-sensitive circadian clock of the enteric bacterium Enterobacter aerogenes. Gut Microbes. 7: 424-427. DOI: 10.1080/19490976. 2016.1208892.
107. Yin J, Li Y, Han H, Chen S, Gao J, Liu G, Wu X, Deng J, Yu Q, Huang X, Fang R (2018) Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high fat diet fed mice. J. Pineal Res. 65: e12524. DOI: 10.1111/jpi.12524.
108. Zhu D, Ma Y, Ding S, Jiang H, Fang J (2018) Effects of melatonin on intestinal microbiota and oxidative stress in colitis mice. Biomed. Res. 2018: 2607679. DOI: 10.1155/2018/2607679.
109. Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T and Yin Y (2018) Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 8: 13. DOI: 10.3389/fcimb.2018.00013.
110. Chojnacki C, Wiśniewska-Jarosińska M, Kulig G, Majsterek I, Reiter RJ, Chojnacki J (2013) Evaluation of enterochromaffin cells and melatonin secretion exponents in ulcerative colitis. World J. Gastroenterol. 19: 3602-3607. DOI: 10.3748/wjg.v19. i23.3602.
111. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51: 1–16. DOI: 10.1111/j.1600-079X.2011.00916.x.
112. Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int. J. Mol. Sci.14: 8638–8683. DOI: 10.3390/ijms14048638.
113. Dong Y, Fan C, Hu W, Jiang S, Ma Z, Yan X, Deng C, Di S, Xin Z, Wu G, Yang Y, Reiter RJ, Liang G (2016) Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signalling. J. Pineal Res. 60: 253–262. DOI: 10.1111/jpi.12300.
114. Reiter RJ, Tan DX, Acuna-Castroviejo D, Burkhardt S, Karbownik M (2000) Melatonin: Mechanisms and actions as an antioxidant. Curr. Top. Biophys. 24: 171–183.
115. Vriend J, Reiter RJ (2014) Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci.115: 8–14. DOI: 10.1016/j.lfs.2014.08.024.
116. Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, Cao Z, Cheng Y, Wang BN, Wang Y (2013) Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF-kB system in high-fat-fed rabbits. J. Pineal Res. 55: 388–398. DOI: 10.1111/jpi.12085.
117. Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S (2017) Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacol. 25: 403-413. DOI 10.1007/s10787-017-0332-5.
118. Akinrinmade FJ, Akinrinde AS, Amid A (2016) Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: modulatory roles of melatonin and flavonoid-rich fractions from Chromolenaodorata. Mycotoxin Res. 32: 53-60.DOI 10.1007/s12550-016-0239-9.
119. Carrillo-Vico A, García-Mauriño S, Calvo JR, Guerrero JM (2003) Melatonin counteracts the inhibitory effect of PGE2 on IL-2 production in human lymphocytes via its mt1 membrane receptor. FASEB J. 17: 755–757.
120. Maldonado MD, García-Moreno H, González-Yanes C, Calvo JR (2016) Possible involvement of the inhibition of NF-κB factor in anti-inflammatory actions that melatonin exerts on mast cells. J. Cell. Biochem.1 17 (8):1926-33. DOI: 10.1002/jcb.25491.
121. Reiter RJ (2000) Melatonin: Lowering the high price of free radicals. News Physiol. Sci. 15: 246–250.
122. Hardeland R, Cardinali DP, Srinivasan V, SpenceDW, Brown GM, Pandi-Perumal SR, (2011) Melatonin—a pleiotropic, orchestrating regulator molecule. Progress Neurobiol. 93: 350–384.
123. Favero G, Franceschetti L, Bonomini F, Rodella LF, Rezzani R (2017) Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017: 1835195. https://doi.org/10.1155/2017/1835195.
124. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian D (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. DOI: 10.1093/cid/ciaa248.
125. Neurath MF (2020) COVID-19 and immunomodulation in IBD. Gut. 69: 1335–1342. DOI: 10.1136/gutjnl-2020-321269.
126. Santaolalla R, Fukata M, Abreu MT (2011) Innate immunity in the small intestine. Curr. Opin. Gastroenterol.27 (2): 125–131. DOI:10.1097/MOG.0b013e3283438dea.
127. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. https://doi.org/10.1007/s11427-020-1643-8.
128. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H (2020) Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 158 (6): 1831–1833. DOI: https://doi.org/10.1053/j.gastro.2020.02.055.
129. Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ (2003) Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300: 1584-1587. DOI: 10.1126/science.1084677.
130. Kim JG, Lee SJ, Kagnoff MF (2004) Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by Toll-like receptors. Infect. Immun. 72: 1487–1495. DOI: 10.1128/IAI.72.3.1487-1495.2004.
131. Pedra JH, Cassel SL, Sutterwala FS (2009) Sensing pathogens and danger signals by the inflammasome. Curr. Opin. Immunol. 21: 10–16. DOI: 10.1016/j.coi.2009.01.006.
132. Stutz A, Golenbock DT, Latz E (2009) Inflammasomes: too big to miss. J. Clin. Invest. 119: 3502–3511. DOI: 10.1172/JCI40599.
133. Zhang R, Wanga X, Nia L, Dia X, Maa B, Niu S, Liua C, Reiter RJ (2020) COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 250: 117583. https://doi.org/10.1016/j.lfs.2020.117583.
134. Gu J, Korteweg C (2007) Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 170 (4): 1136–1147. DOI: 10.2353/ajpath.2007.061088.
135. Gu J, Gong EC, Zhang B, Zheng J, Gao ZF, Zhong YF, Zou WZ, Zhan J, Wang SL, Xie ZG, Zhuang H, Wu BQ, Zhong HH, Shao HQ, Fang WG, Gao DX, Pei F, Li XW, He ZP, Xu DZ, Shi XY, Anderson VM, Leong ASY (2005) Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 202: 415-424. DOI: 10.1084/jem.20050828.
136. Shi X, Gong E, Gao D, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Wu B, Fang W, Liao S, Wang S, Xie Z, Lu M, Hou L, Zhong H, Shao H, Li N, Liu C, Pei F, Yang J, Wang Y, Han Z, Shi X, Zhang Q, You J, Zhu X, Gu J (2005) Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am. J. Gastroenterol. 100: 169-176. DOI: 10.1111/j.1572-0241.2005.40377.x.
137. Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ (2003) Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125: 1011–1017.DOI: 10.1016/s0016-5085(03)01215-0.
138. He L, Ding Y, Zhang Q, Che X, He Y, Shen H, Wang H, Li Z, Zhao L, Geng J, Deng Y, Yang L, Li J, Cai J, Qiu L, Wen K, Xu X, Jiang S (2006) Expression of elevated levels of pro-inflammatory cytokines in SARSCoV- infected ACE2(+) cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 210: 288–297. DOI: 10.1002/path.2067.
139. Miller SC, Pandi-Perumal SR, Esquifino AI, Cardinali DP, Maestroni GJM (2006) The role of melatonin in immuno-enhancement: potential application in cancer. Int. J. Exp. Pathol. 87: 81–87. https://doi.org/10.1111/j.0959-9673.2006.00474.x.
140. Kaur C, Ling EA (1999) Effects of melatonin on macrophages/microglia in postnatal rat brain. J. Pineal Res. 26: 158–168. https://doi.org/10.1111/j.1600-079x.1999.tb00578.x.
141. Park et al., 2015 Park YS, Chung SH, Lee SK, Kim JH, Kim JB, Kim TK, Kim DS, Baik HW (2015) Melatonin improves experimental colitis with sleep deprivation. Int. J. Mol. Med. 35: 979-986. DOI: 10.3892/ijmm.2015.2080.
142. Sanchez-Lopez AL, Ortiz GG, Pacheco-Moises FP, Mireles-Ramirez MA, Bitzer-Quintero OK, Delgado-Lara DLC, Ramirez-Jirano LJ, Velazquez- Brizuela IE (2018) Efficacy of melatonin on serum pro-inflammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch. Med. Res. 49: 391–398. https://doi.org/10.1016/j.arcmed.2018.12.004.
143. Bazyar H, Gholinezhad H, Moradi L, Salehi P, Abadi F, Ravanbakhsh M, Zare Javid A (2019) The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: a double-blind, placebo-controlled trial. Inflammopharmacol. 27: 67–76. https://doi.org/10.1007/s10787-018-0539-0.
144. Tahan et al., 2011 Tahan G, Gramignoli R, Marongiu F, Aktolga S, Cetinkaya A, Tahan V, Dorko K (2011) Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig. Dis. Sci. 56: 715-720. DOI: 10.1007/s10620-010-1364-5.
145. Volt H, García JA, Doerrier C, Díaz-Casado ME, Guerra-Librero A, López LC, Escames G, Tresguerres JA, Acuña-Castroviejo D (2016) Same molecule but different expression: aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 60: 193–205. DOI: 10.1111/jpi.12303.
146. Zarezadeh M, Khorshidi M, Emami M, Janmohammadi P, Kord-Varkaneh H, Mousavi SM, Mohammed SH, Saedisomeolia A, Alizadeh S (2019) Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials, Eur. J. Nutr. https://doi.org/10.1007/s00394-019-02123-0.
147. Bourne RS, Mills GH, Minelli C (2008) Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial, Crit. Care. 12: R52. https://doi.org/10.1186/cc6871.
148. Mistraletti G, Umbrello M, Sabbatini G, Miori S, Taverna M, Cerri B, Mantovani ES, Formenti P, SpanuP, D’Agostino A, Salini S, Morabito A, Fraschini F, Reiter RJ, Iapichino G (2015) Melatonin reduces the need for sedation in ICU patients: a randomized controlled trial. Minerva Anestesiol. 81: 1298–1310.
149. Andersen LPH, Gogenur I, Rosenberg J, Reiter RJ (2016) The safety of melatonin in humans. Clin. Drug Investig. 36: 169–175. https://doi.org/10.1007/s40261-015-0368-5.
150. Nordlund JJ, Lerner AB (1977) The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clin. Endocrinol. Metab. 45: 768–774. https://doi.org/10.1210/jcem-45-4-768.
151. Sun CK, Lee FY, Kao YH, Chiang HJ, Sung PH, Tsai TH, Lin YC, Leu S, Wu YC, Lu HI, Chen YL, Chung SY, Su HL, Yip HK (2015) Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J. Pineal Res. 58: 137–150. https://doi.org/10.1111/jpi.12199
152. Reiter RJ, Abreu-Gonzalez P, Marik PE, Dominguez-Rodriguez A (2020) Therapeutic algorithm for use of melatonin in patients with COVID-19. Front Med. 7: 226. DOI: 10.3389/fmed.2020.00226.
153. Giménez VMM, Inserra F, Tajer CD, Mariani J, Ferder L, Reiter RJ, Manucha W (2020) Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 254: 117808. DOI: 10.1016/j.lfs.2020.117808.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


