Melatonin, the advance-guard in oxidative myocardial assault instigated by exercise stress: a physiological and biochemical insight
Melatonin in exercise-induced cardiac stress
Abstract
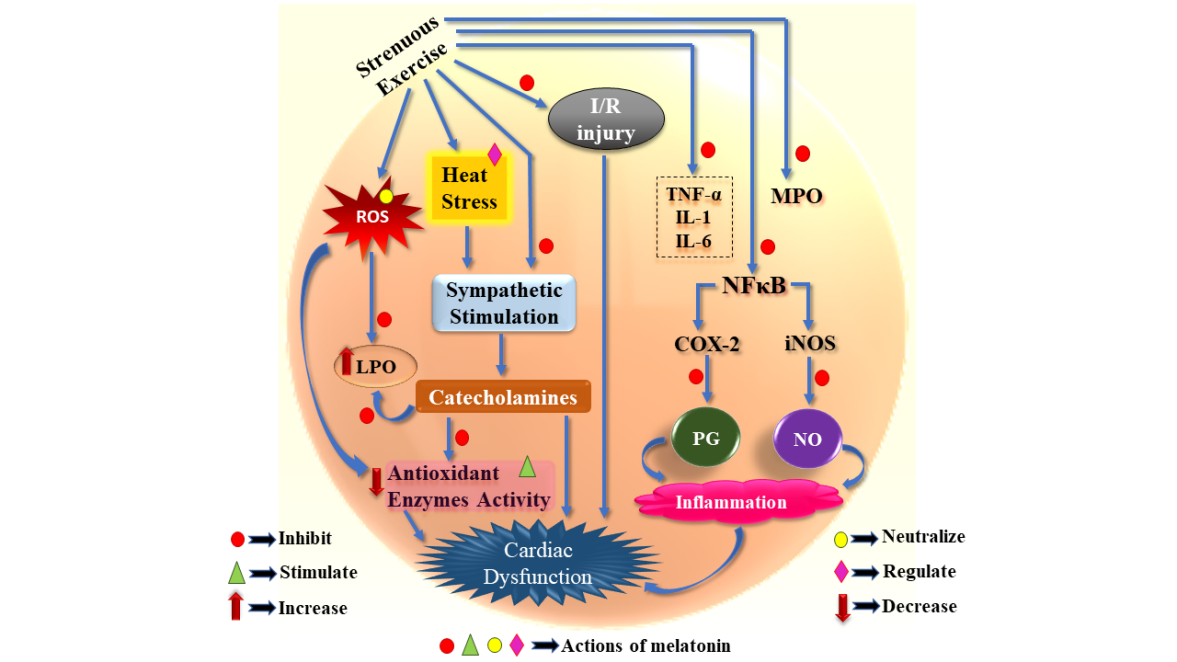
Exercise conducted at an optimum training load is usually beneficial for the overall health of an individual. However, an unaccustomed intense exercise carried out by untrained individuals or elite athletes during over-training and/or competition-related stress often bear inevitable cardiovascular risks. Although many alterations occurring in the cardiovascular system during exercise are the results of training adaptations, sudden cardiovascular deaths reported in competitive athletes is a matter of grave concern. Several oxidative biomarkers that depict the underlying structural and functional impairment of the myocardial tissue have been identified in the individuals subjected to extensive exercise. The exercise-mediated cardiomyopathy is free radical related and also associated with pro-inflammatory response. In this review we will highlight the possible role of melatonin in obviating irrevocable oxidative cardiovascular injury triggered by extensive exercise stress. Melatonin effectively reduces exercise-induced lipid peroxidation, restores natural cellular antioxidant pool and supresses the innate immune cascade reaction that, otherwise, jeopardize cardiovascular integrity. Melatonin blocks the IKK/IκB/NFκB signaling as well as suppress iNOS and COX-2 mediated inflammation in cardiac tissue. In addition, melatonin reduces blood lactate accumulation and accelerates glucose utilization, thereby, promoting energy metabolism in athletes during their training and competition. Physical exertion associated overheating and the resultant sympathetic outflow impede cardiovascular homeostasis. Melatonin not only attenuates the sympathomedullary stimulation but also protects the cardiac cells from the cytotoxic effect of catecholamines. The available information regarding the efficacy of melatonin in amelioration of exercise-driven oxidative insult in cardiac tissue has been discussed and summarized.
References
2. Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4: 180-183. DOI: 10.1016/j.redox.2015.01.002.
3. Higuchi M, Cartier LJ, Chen M, Holloszy JO (1985) Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J. Gerontol. 40: 281-286. DOI: 10.1093/geronj/40.3.281.
4. Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ (1985) Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J. Appl. Physiol. 59: 1298-1303. DOI: 10.1152/jappl.1985.59.4.1298.
5. Sen CK, Marin EI, Kretzschmar MI, Hanninen OS (1992) Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J. Appl. Physiol. 73: 1265-1272. DOI: 10.1152/jappl.1992.73.4.1265.
6. Booth FW, Roberts CK, Laye MJ (2011) Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2: 1143-1211. DOI: 10.1002/cphy.c110025.
7. Leaf DA, Kleinman MT, Hamilton M, Barstow TJ (1997) The effect of exercise intensity on lipid peroxidation. Med. Sci. Sports Exerc. 29: 1036-1039. DOI: 10.1097/00005768-199708000-00008.
8. Marzatico F, Pansarasa O, Bertorelli L, Somenzini L, Della GV (1997) Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J. Sport Med. Phys. Fit. 37: 235-239.
9. Alessio HM, Goldfarb AH, Cao G (1997) Exercise-induced oxidative stress before and after vitamin C supplementation. Int. J. Sport. Nutr. Exerc. Metab. 7: 1-9. DOI: 10.1123/ijsn.7.1.1.
10. Niess AM, Hartmann A, Grünert-Fuchs M, Poch B, Speit G (1996) DNA damage after exhaustive treadmill running in trained and untrained men. Int. J. Sports Med. 17: 397-403. DOI: 10.1055/s-2007-972868.
11. Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL (1978) Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. 45: 927-932. DOI: 10.1152/jappl.1978.45.6.927.
12. Powers SK, Radak Z, Ji LL (2016) Exercise‐induced oxidative stress: past, present and future. J. Physiol. 594: 5081-5092. DOI: 10.1113/JP270646.
13. Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE (2000) Triggering of sudden death from cardiac causes by vigorous exertion. N. Engl. J. Med. 343: 1355-1361. DOI: 10.1056/NEJM200011093431902.
14. Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ (2007) Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 116: 572-584. DOI: 10.1161/CIRCULATIONAHA.107.185214.
15. Michailidis Y, Jamurtas AZ, Nikolaidis MG, Fatouros IG, Koutedakis Y, Papassotiriou I, Kouretas D (2007) Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med. Sci. Sport. Exer. 39: 1107-1113. DOI: 10.1249/01.mss.0b013e318053e7ba.
16. Brady PS, Brady LJ, Ullrey DE (1979) Selenium, vitamin E and the response to swimming stress in the rat. Nutr. J. 109: 1103-1109. DOI: 10.1093/jn/109.6.1103.
17. Coombes JS, Rowell B, Dodd SL, Demirel HA, Naito H, Shanely AR, Powers SK (2002) Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur. J. Appl. Physiol. 87: 272-277. DOI: 10.1007/s00421-002-0631-3.
18. Huether G (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49: 665-670. DOI: 10.1007/BF01923948.
19. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253-278. DOI: 10.1111/jpi.12360.
20. Ochoa JJ, Díaz‐Castro J, Kajarabille N, García C, Guisado IM, De Teresa C, Guisado R (2011) Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 51: 373-380. DOI: 10.1111/j.1600-079X.2011.00899.x.
21. Borges LD, Dermargos A, Junior EP, Weimann E, Lambertucci RH, Hatanaka E (2015) Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J. Pineal Res. 58: 166-172. DOI: 10.1111/jpi.12202.
22. Leonardo-Mendonça RC, Ocaña-Wilhelmi J, de Haro T, de Teresa-Galván C, Guerra-Hernández E, Rusanova I, Fernández-Ortiz M, Sayed RK, Escames G, Acuña-Castroviejo D (2017) The benefit of a supplement with the antioxidant melatonin on redox status and muscle damage in resistance-trained athletes. Appl. Physiol. Nutr. Metab. 42: 700-707. DOI: 10.1139/apnm-2016-0677.
23. Jiki Z, Lecour S, Nduhirabandi F (2018) Cardiovascular benefits of dietary melatonin: a myth or a reality? Front. Physiol. 9: 528. DOI: 10.3389/fphys.2018.00528.
24. Cimen B, Uz A, Cetin I, Cimen L, Cetin A (2017) Melatonin supplementation ameliorates energy charge and oxidative stress induced by acute exercise in rat heart tissue. Acta Cardiol. Sin. 33: 530-538. DOI: 10.6515/acs20170331a.
25. Veneroso C, Tuñón MJ, González‐Gallego J, Collado PS (2009) Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal Res. 47: 184-191. DOI: 10.1111/j.1600-079X.2009.00699.x.
26. Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR (1986) Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ. Res. 58: 281-291. DOI: 10.1161/01.res.58.2.281.
27. Ekblom B, Hermansen L (1968) Cardiac output in athletes. J. Appl. Physiol. 25: 619-625. DOI: 10.1152/jappl.1968.25.5.619.
28. Zavorsky GS (2000) Evidence and possible mechanisms of altered maximum heart rate with endurance training and tapering. Sports Med. 29: 13-26. DOI: 10.2165/00007256-200029010-00002.
29. Hellsten Y, Nyberg M (2011) Cardiovascular adaptations to exercise training. Compr. Physiol. 6: 1-32. DOI: 10.1002/cphy.c140080.
30. Thompson PD (2004) Historical concepts of the athlete's heart. Med. Sci. Sports Exerc. 36: 363-370. DOI: 10.1249/01.MSS.0000117117.67849.F6.
31. Roskamm H, Reindell H, Musshoff K, Koenig K (1961) Relations between heart size and physical efficiency in male and female athletes in comparison with normal male and female subjects. III. Archiv. Fur. Kreislaufforschung 35: 67-102.
32. Fagard R (2003) Athlete’s heart. Heart 89: 1455-1461. DOI: 10.1136/heart.89.12.1455.
33. Estes NA, Link MS, Homoud M, Wang PJ (2001) Electrocardiographic variants and cardiac rhythm and conduction disturbances in the athlete. Exercise and sports cardiology (New York: McGraw-Hill), pp 211-232.
34. Simons SM, Berry J, Bartsokas TW (1993) Preventing sudden death: the role of automated defibrillators. Phys. Sports Med. 21: 53-59. DOI: https://doi.org/10.1080/00913847.1993.11710429.
35. Maron BJ, Thompson PD, Puffer JC, McGrew CA, Strong WB, Douglas PS, Clark LT, Mitten MJ, Crawford MH, Atkins DL, Driscoll DJ (1996) Cardiovascular preparticipation screening of competitive athletes: a statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 94: 850-856. DOI: 10.1161/01.cir.94.4.850.
36. Maron BJ (1998) Hypertrophic cardiomyopathy as a cause of sudden death in the young competitive athlete. Sudden Cardiac Death in the Athlete (Futura Publishing, Armonk), pp 301-317. DOI: https://doi.org/10.1016/S0146-2806(98)80008-X.
37. Convertino VA, Brock PJ, Keil LC, Bernauer EM, Greenleaf JE (1980) Exercise training-induced hypervolemia: role of plasma albumin, renin, and vasopressin. J. Appl. Physiol. 48: 665-669. DOI: 10.1152/jappl.1980.48.4.665.
38. Convertino VA, Keil LC, Bernauer EM, Greenleaf JE (1981) Plasma volume, osmolality, vasopressin, and renin activity during graded exercise in man. J. Appl. Physiol. 50: 123-128. DOI: 10.1152/jappl.1981.50.1.123.
39. Mairbäurl H (2013) Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 4: 332. DOI: 10.3389/fphys.2013.00332.
40. Kim D, Ha JW (2016) Hypertensive response to exercise: mechanisms and clinical implication. J. Clin. Hypertens. 22: 17. DOI: 10.1186/s40885-016-0052-y.
41. de Miranda Rohlfs ICP, de Mara LS, de Lima WC, de Carvalho T (2005) Relationship of the overtraining syndrome with stress, fatigue, and serotonin. Rev. Bras. Med. Esporte. 11: 333-337. DOI: https://doi.org/10.1590/S1517-86922005000600012.
42. van Paridon KN, Timmis MA, Nevison CM, Bristow M (2017) The anticipatory stress response to sport competition; a systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport Exerc. Med. 3: e000261. DOI: 10.1136/bmjsem-2017-000261.
43. Henry JP (1992) Biological basis of the stress response. Integr. Psychol. Behav. Sci. 27: 66-83. DOI: 10.1007/BF02691093.
44. Smelik PG (1985) Stress and hormones. Organorama 22: 16-18.
45. de Boer SF, De Beun R, Slangen JL, Van der Gugten J (1990) Dynamics of plasma catecholamine and corticosterone concentrations during reinforced and extinguished operant behavior in rats. Physiol. Behav. 47: 691-698. DOI: https://doi.org/10.1016/0031-9384(90)90079-J.
46. Selye H. (1936) A syndrome produced by diverse noxious agents. Nature 138: 32-34. DOI:10.1038/138032a0.
47. Lucía A, Diaz B, Hoyos J, Fernandez C, Villa G, Bandres F, Chicharro JL (2001) Hormone levels of world class cyclists during the Tour of Spain stage race. Brit. J. Sport Med. 35: 424-430. DOI: 10.1136/bjsm.35.6.424.
48. Stacchiotti A, Favero G, Rodella LF (2020) Impact of melatonin on skeletal muscle and exercise. Cells 9: 288. DOI: 10.3390/cells9020288.
49. Pereira EJ, Smolko CM, Janes KA (2016) Computational models of reactive oxygen species as metabolic byproducts and signal-transduction modulators. Front. Pharmacol. 7: 457. DOI: 10.3389/fphar.2016.00457.
50. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A (2017) Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017: 8416763. DOI: 10.1155/2017/8416763.
51. Kelkar G, Subhadra K, Chengappa RK (2008) Effect of antioxidant supplementation on hematological parameters, oxidative stress and performance of Indian athletes. J. Hum. Ecol. 24: 209-213. DOI: 10.1080/09709274.2008.11906116.
52. Ribeiro-Samora GA, Rabelo LA, Ferreira AC, Favero M, Guedes GS, Pereira LS, Parreira VF, Britto RR (2017) Inflammation and oxidative stress in heart failure: effects of exercise intensity and duration. Braz. J. Med. Biol. Res. 50: e6393. DOI: 10.1590/1414-431X20176393.
53. König D, Neubauer O, Nics L, Kern N, Berg A, Bisse E, Wagner KH (2007) Biomarkers of exercise-induced myocardial stress in relation to inflammatory and oxidative stress. Exerc. Immunol. Rev. 13: 15-36.
54. Mair J, Thome-Kromer B, Wagner I, Lechleitner P, Dienstl F, Puschendorf B, Michel G (1994) Concentration time courses of troponin and myosin subunits after acute myocardial infarction. Coron. Artery Dis. 5: 865-872.
55. Neumayr G, Gaenzer H, Pfister R, Sturm W, Schwarzacher SP, Eibl G, Mitterbauer G, Hoertnagl H (2001) Plasma levels of cardiac troponin I after prolonged strenuous endurance exercise. Am. J. Cardiol. 87: 369-371. DOI: 10.1016/s0002-9149(00)01382-5.
56. Wu AH, Feng YJ, Moore R, Apple FS, McPherson PH, Buechler KF, Bodor G, Standardization CC (1998) Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. Clin. Chem. 44:1198-1208.
57. Ooi DS, Isotalo PA, Veinot JP (2000) Correlation of antemortem serum creatine kinase, creatine kinase-MB, troponin I, and troponin T with cardiac pathology. Clin. Chem. 46:338-344.
58. Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R (1999) National academy of clinical biochemistry standards of laboratory practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin. Chem. 45:1104-1121.
59. Kato M, Kinugawa T, Ogino K, Endo A, Osaki S, Igawa O, Hisatome I, Shigemasa C (2000) Augmented response in plasma brain natriuretic peptide to dynamic exercise in patients with left ventricular dysfunction and congestive heart failure. J. Intern. Med. 248: 309-315. DOI: 10.1046/j.1365-2796.2000.00736.x.
60. Sagnella GA (2001) Measurement and importance of plasma brain natriuretic peptide and related peptides. Ann. Clin. Biochem. 38: 83-93. DOI: 10.1258/0004563011900317.
61. McNairy M, Gardetto N, Clopton P, Garcia A, Krishnaswamy P, Kazanegra R, Ziegler M, Maisel AS (2002) Stability of B-type natriuretic peptide levels during exercise in patients with congestive heart failure: implications for outpatient monitoring with B-type natriuretic peptide. Am. Heart J. 143:406-411. DOI: 10.1067/mhj.2002.120148.
62. Friedl W, Mair J, Thomas S, Pichler M, Puschendorf B (1999) Relationship between natriuretic peptides and hemodynamics in patients with heart failure at rest and after ergometric exercise. Clinica chimica acta 281: 121-126. DOI: 10.1016/s0009-8981(98)00217-4.
63. Ohba H, Takada H, Musha H, Nagashima J, Mori N, Awaya T, Omiya K, Murayama M (2001) Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. Am. Heart J. 141:751-758. DOI: 10.1067/mhj.2001.114371.
64. Jacob R, Khan M (2018) Cardiac Biomarkers: What Is and What Can Be. Ind. J. Cardiovascul. Dis. in Women 3: 240-244. DOI: 10.1055/s-0039-1679104.
65. Smith JE, Garbutt G, Lopes P, Pedoe DT (2004) Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Brit. J. Sport Med. 38:292-294. DOI: 10.1136/bjsm.2002.002873.
66. Rahnama N, Faramarzi M, Gaeini AA (2011) Effects of intermittent exercise on cardiac troponin I and creatine kinase-MB. Int. J. Prev. Med. 2:20.
67. Scharhag J, George K, Shave R, Urhausen A, Kindermann W (2008) Exercise-associated increases in cardiac biomarkers. Med. Sci. Sports Exerc .40: 1408-1415. DOI: 10.1249/MSS.0b013e318172cf22.
68. Shave R, George K, Gaze D (2007) The influence of exercise upon cardiac biomarkers: a practical guide for clinicians and scientists. Curr. Med. Chem. 14: 1427-1436. DOI: 10.2174/092986707780831177.
69. Apple FS, Quist HE, Otto AP, Mathews WE, Murakami MM (2002) Release characteristics of cardiac biomarkers and ischemia-modified albumin as measured by the albumin cobalt-binding test after a marathon race. Clin. Chem. 48: 1097-1100. DOI: 10.1093/clinchem/48.7.1097.
70. Middleton N, Shave R, George K, Whyte G, Forster J, Oxborough D, Gaze D, Collinson P (2006) Novel application of flow propagation velocity and ischaemia‐modified albumin in analysis of postexercise cardiac function in man. Exp. Physiol. 91: 511-519. DOI: 10.1113/expphysiol.2005.032631.
71. Dawson E, George K, Shave R, Whyte G, Ball D (2003) Does the human heart fatigue subsequent to prolonged exercise? Sports Med. 33: 365-380. DOI: 10.2165/00007256-200333050-00003.
72. La Gerche A, Inder WJ, Roberts TJ, Brosnan MJ, Heidbuchel H, Prior DL (2015) Relationship between inflammatory cytokines and indices of cardiac dysfunction following intense endurance exercise. PLoS One. 10: e0130031. DOI: 10.1371/journal.pone.0130031.
73. Melanson SE, Green SM, Wood MJ, Neilan TG, Lewandrowski EL (2006) Elevation of myeloperoxidase in conjunction with cardiac-specific markers after marathon running. Am. J. Clin. Pathol. 126: 888-893. DOI: 10.1309/1D62H6KRFTVQRJ0A.
74. Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, Winterbourn CC (2007) Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 49: 1993-2000. DOI: 10.1016/j.jacc.2007.02.040.
75. García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ, González-Gallego J (2007) The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 557: 221-229. DOI: 10.1016/j.ejphar.2006.11.014.
76. Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, González-Gallego J (2004) Quercetin prevents oxidative stress and NF-κB activation in gastric mucosa of portal hypertensive rats. Biochem. Pharmacol. 68: 1939-1946. DOI: 10.1016/j.bcp.2004.07.016.
77. Alonso M, Collado PS, González‐Gallego J (2006) Melatonin inhibits the expression of the inducible isoform of nitric oxide synthase and nuclear factor kappa B activation in rat skeletal muscle. J. Pineal Res. 41: 8-14. DOI: 10.1111/j.1600-079X.2006.00323.x.
78. Wernerman J (2012) Micronutrients against oxidative stress-time for clinical recommendations? Crit. Care 16: 124. DOI: 10.1186/cc11319.
79. Braakhuis AJ, Hopkins WG (2015) Impact of dietary antioxidants on sport performance: a review. Sports Med. 45: 939-955. DOI: 10.1007/s40279-015-0323-x.
80. Kreher JB, Schwartz JB (2012) Overtraining syndrome: a practical guide. Sports Health 4: 128-138. DOI: 10.1177/1941738111434406.
81. Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J (2008) Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 87: 142-149. DOI: 10.1093/ajcn/87.1.142.
82. Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 106: 8665-8670. DOI: 10.1073/pnas.0903485106.
83. Novelli GP, Bracciotti G, Falsini S (1990) Spin-trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radic. Biol. Med. 8: 9-13. DOI: https://doi.org/10.1016/0891-5849(90)90138-9.
84. Lawrence JD, Bower RC, Riehl WP, Smith JL (1975) Effects of alpha-tocopherol acetate on the swimming endurance of trained swimmers. Am. J. Clin. Nutr. 28: 205-208. DOI: 10.1093/ajcn/28.3.205.
85. Romano-Ely BC, Todd MK, Saunders MJ, Laurent TS (2006) Effect of an isocaloric carbohydrate-protein-antioxidant drink on cycling performance. Med. Sci. Sports Exerc. 38: 1608-1616. DOI: 10.1249/01.mss.0000229458.11452.e9.
86. Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Østgaard HN, Buer C (2014) Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double‐blind, randomised, controlled trial. J. Physiol. 592: 1887-1901. DOI: 10.1113/jphysiol.2013.267419.
87. Kobayaski, Y (1974) Effect of vitamin E on aerobic work performance in man during acute exposure to hypoxic hypoxia (Doctoral dissertation, University of New Mexico).
88. Ilavazhagan G, Bansal A, Prasad D, Thomas P, Sharma SK, Kain AK, Kumar D, Selvamurthy W (2001) Effect of vitamin E supplementation on hypoxia-induced oxidative damage in male albino rats. Aviat. Space Environ. Med. 72: 899-903.
89. Ulusoy HG, Sanlier N (2019) A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 1: 1-4. DOI: 10.1080/10408398.2019.1683810.
90. Davis JM, Murphy EA, Carmichael MD, Davis B (2009) Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296: R1071-1077. DOI: 10.1152/ajpregu.90925.2008.
91. Nieman DC (2007) Marathon training and immune function. Sports Med. 37: 412-415. DOI: 10.2165/00007256-200737040-00036.
92. Murase T, Haramizu S, Ota N, Hase T (2009) Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology 10: 423-434. DOI: 10.1007/s10522-008-9177-z.
93. Xiao NN (2015) Effects of resveratrol supplementation on oxidative damage and lipid peroxidation induced by strenuous exercise in rats. Biomol. Ther. 23: 374-378. DOI: 10.4062/biomolther.2015.015.
94. Hart N, Sarga L, Csende Z, Koch LG, Britton SL, Davies KJ, Radak Z (2014) Resveratrol attenuates exercise-induced adaptive responses in rats selectively bred for low running performance. Dose-Response. 12: 57-71. DOI: 10.2203/dose-response.13-010.Radak.
95. Gliemann L, Schmidt JF, Olesen J, Biensø RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y (2013) Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 591: 5047-5059. DOI: 10.1113/jphysiol.2013.258061.
96. Motterlini R, Foresti R, Bassi R, Green CJ (2000) Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 28: 1303-1312. DOI: 10.1016/s0891-5849(00)00294-x.
97. Sharma OP (1976) Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 25: 1811. DOI: 10.1016/0006-2952(76)90421-4.
98. Abe Y, Hashimoto SH, Horie T (1999) Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 39: 41-47. DOI: 10.1006/phrs.1998.0404.
99. Roohi BN, Moradlou AN, Bolboli L (2017) Influence of curcumin supplementation on exercise-induced oxidative stress. Asian J. Sports Med. 8: e35776. DOI: 10.5812/asjsm.35776.
100. Davis JM, Murphy EA, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS, Gangemi JD, Ghaffar A, Mayer EP (2007) Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292: R2168-2173. DOI: 10.1152/ajpregu.00858.2006.
101. Takahashi M, Suzuki K, Kim HK, Otsuka Y, Imaizumi A, Miyashita M, Sakamoto S (2014) Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 35: 469-475. DOI: 10.1055/s-0033-1357185.
102. Basham SA, Waldman HS, Krings BM, Lamberth J, Smith JW, McAllister MJ (2019) Effect of Curcumin Supplementation on Exercise-Induced Oxidative Stress, Inflammation, Muscle Damage, and Muscle Soreness. J. Diet. Suppl. 26: 1-4. DOI: 10.1080/19390211.2019.1604604.
103. Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ (2004) N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J. Appl. Physiol. 97: 1477-1485. DOI: 10.1152/japplphysiol.00371.2004.
104. McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X (2006) N‐acetylcysteine attenuates the decline in muscle Na+, K+‐pump activity and delays fatigue during prolonged exercise in humans. J. Physiol. 576: 279-288. DOI: 10.1113/jphysiol.2006.115352.
105. Kawamura T, Muraoka I (2018) Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants 7: 119. DOI: 10.3390/antiox7090119.
106. Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958) Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 80: 2587. DOI: https://doi.org/10.1021/ja01543a060.
107. Lerner AB, Case JD, Heinzelmann RV (1959) Structure of melatonin. J. Am. Chem. Soc. 81: 6084-6085. DOI: https://doi.org/10.1021/ja01531a060.
108. Cardinali, D. P., Pandi-Perumal, S. R., & Niles, L. P (2008) Melatonin and its receptors: biological function in circadian sleep-wake regulation (Cambridge University Press, Cambridge), pp 283-314. DOI: 10.1017/CBO9780511541674.011.
109. Korkmaz A, Topal T, Tan DX, Reiter RJ (2009) Role of melatonin in metabolic regulation. Rev. Endocr. Metab. Disord. 10: 261-270. DOI: 10.1007/s11154-009-9117-5.
110. Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM (2009) Melatonin and reproduction revisited. Biol. Reprod. 81: 445-456. DOI: 10.1095/biolreprod.108.075655.
111. Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res.2: 158-184. DOI: 10.32794/mr11250027.
112. Pal PK, Sarkar S, Chattopadhyay A, Tan DX, Bandyopadhyay D (2019) Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals. Melatonin Res.2: 61-82. DOI: 10.32794/mr11250041.
113. Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L (2010) Beneficial effects of melatonin in cardiovascular disease. Ann. Med. 42: 276-285. DOI: 10.3109/07853890903485748.
114. Lee JG, Woo YS, Park SW, Seog DH, Seo MK, Bahk WM (2019) The neuroprotective effects of melatonin: Possible role in the pathophysiology of neuropsychiatric disease. Brain Sci. 9: 285. DOI: 10.3390/brainsci9100285.
115. Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D (2006) The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs.7: 423.
116. Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF, Xu K (2017) Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 18: 843. DOI: 10.3390/ijms18040843.
117. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra MC, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181-197. DOI: 10.2174/1568026023394443.
118. Tan DX, Manchester LC, Hardeland R, Lopez‐Burillo S, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res .34: 75-78. DOI: 10.1034/j.1600-079x.2003.02111.x.
119. Cipolla-Neto J, Amaral FG (2018) Melatonin as a hormone: new physiological and clinical insights. Endocr. Rev. 39: 990-1028. DOI: 10.1210/er.2018-00084.
120. Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: a never‐ending interaction of melatonin with reactive oxygen and nitrogen species?. J. Pineal Res. 42: 28-42. DOI: 10.1111/j.1600-079X.2006.00407.x.
121. Galano A, Medina ME, Tan DX, Reiter RJ (2015) Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis. J. Pineal Res. 58: 107-116. DOI: 10.1111/jpi.12196.
122. Pariente R, Bejarano I, Espino J, Rodríguez AB, Pariente JA (2017) Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother. Pharmacol. 80: 985-998. DOI: 10.1007/s00280-017-3441-3.
123. Reiter RJ, Tan DX, Manchester LC, Terron MP, Flores LJ, Koppisepi S (2007) Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv. Med. Sci. 52: 11-28.
124. Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Hölzenbein T, Markovic O, Leibetseder VJ, Strauss‐Blasche G, Marktl W (2003) The melatonin receptor subtype MT2 is present in the human cardiovascular system. J. Pineal Res. 35: 40-44. DOI: 10.1034/j.1600-079x.2003.00051.x.
125. Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ (2012) Melatonin and cardiovascular disease: myth or reality? Rev. Esp. Cardiol.(English Edition). 65: 215-218. DOI: 10.1016/j.recesp.2011.10.009.
126. Pandi-Perumal SR, BaHammam AS, Ojike NI, Akinseye OA, Kendzerska T, Buttoo K, Dhandapany PS, Brown GM, Cardinali DP (2017) Melatonin and human cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 22: 122-132. DOI: 10.1177/1074248416660622.
127. Krause DN, Geary GG, Doolen S, Duckles SP (2002) Melatonin and Cardiovascular Function. In Melatonin after Four Decades. (Springer, Boston, MA), pp 299-310. DOI: https://doi.org/10.1007/0-306-46814-X_32.
128. Xia CM, Shao CH, Xin L, Wang YR, Ding CN, Wang J, Shen LL, Li L, Cao YX, Zhu DN (2008) Effects of melatonin on blood pressure in stress‐induced hypertension in rats. Clin. Exp. Pharmacol. Physiol. 35: 1258-1264. DOI: 10.1111/j.1440-1681.2008.05000.x.
129. Prado NJ, Ferder L, Manucha W, Diez ER (2018) Anti-inflammatory effects of melatonin in obesity and hypertension. Curr. Hypertens. Rep. 20: 45. DOI: 10.1007/s11906-018-0842-6.
130. Simko F, Baka T, Krajcirovicova K, Repova K, Aziriova S, Zorad S, Poglitsch M, Adamcova M, Reiter RJ, Paulis L (2018) Effect of melatonin on the renin-angiotensin-aldosterone system in L-NAME-induced hypertension. Molecules 23: 265. DOI: 10.3390/molecules23020265.
131. Diez ER, Renna NF, Prado NJ, Lembo C, Ponce Zumino AZ, Vazquez‐Prieto M, Miatello RM (2013) Melatonin, given at the time of reperfusion, prevents ventricular arrhythmias in isolated hearts from fructose-fed rats and spontaneously hypertensive rats. J. Pineal Res. 55: 166-173. DOI: 10.1111/jpi.12059.
132. Benova T, Viczenczova C, Radosinska J, Bacova B, Knezl V, Dosenko V, Weismann P, Zeman M, Navarova J, Tribulova N (2013) Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can. J. Physiol. Pharmacol. 91: 633-639. DOI: 10.1139/cjpp-2012-0393.
133. Sedova KA, Bernikova OG, Cuprova JI, Ivanova AD, Kutaeva GA, Pliss MG, Lopatina EV, Vaykshnorayte MA, Diez ER, Azarov JE (2019) Association between antiarrhythmic, electrophysiological, and antioxidative effects of melatonin in ischemia/reperfusion. Int. J. Mol. Sci. 20: 6331. DOI: 10.3390/ijms20246331.
134. Prado NJ, Muñoz EM, Farias Altamirano LE, Aguiar F, Ponce Zumino AZ, Sánchez FJ, Miatello RM, Pueyo E, Diez ER (2020) Reperfusion arrhythmias increase after superior cervical ganglionectomy due to conduction disorders and changes in repolarization. Int. J. Mol. Sci. 21: 1804. DOI: 10.3390/ijms21051804.
135. Dominguez-Rodriguez A, Abreu-Gonzalez P, Jose M, Consuegra-Sanchez L, Piccolo R, Gonzalez-Gonzalez J, Garcia-Camarero T, del Mar Garcia-Saiz M, Aldea-Perona A, Reiter RJ, Caballero-Estevez N (2017) Usefulness of early treatment with melatonin to reduce infarct size in patients with ST-segment elevation myocardial infarction receiving percutaneous coronary intervention (From the Melatonin Adjunct in the Acute Myocardial Infarction Treated With Angioplasty Trial). Am. J. Cardiol. 120: 522-526. DOI:10.1016/j.amjcard.2017.05.018.
136. Dominguez-Rodriguez A, Abreu-Gonzalez P, Chen Y (2019) Cardioprotection and effects of melatonin administration on cardiac ischemia reperfusion: Insight from clinical studies. Melatonin Res. 2: 100-105. DOI:https://doi.org/https://doi.org/10.32794/mr11250024.
137. Bojkowski CJ, Arendt J (1990) Factors influencing urinary 6‐sulphatoxymelatonin, a major melatonin metabolite, in normal human subjects. Clin. Endocrinol. 33: 435-444. DOI: 10.1111/j.1365-2265.1990.tb03882.x.
138. Sakotnik A, Liebmann PM, Stoschitzky K, Lercher P, Schauenstein K, Klein W, Eber B (1999) Decreased melatonin synthesis in patients with coronary artery disease. Eur. Heart J. 20: 1314-1317. DOI: 10.1053/euhj.1999.1527.
139. Girotti L, Lago M, Ianovsky O, Carbajales J, Elizari MV, Brusco LI, Cardinali DP (2000) Low urinary 6‐sulphatoxymelatonin levels in patients with coronary artery disease. J. Pineal Res.29: 138-142. DOI: 10.1034/j.1600-079x.2000.290302.x.
140. Dominguez-Rodriguez A, Abreu-Gonzalez P, Piccolo R, Galasso G, Reiter RJ (2016) Melatonin is associated with reverse remodeling after cardiac resynchronization therapy in patients with heart failure and ventricular dyssynchrony. Int. J. Cardiol. 221: 359-363. DOI:10.1016/j.ijcard.2016.07.056.
141. Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ (2010). The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 85 (3): 607-23. doi: 10.1111/j.1469-185X.2009.00118.
142. Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrinol.10: 249. DOI: 10.3389/fendo.2019.00249.
143. Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP (2005) Exercise and the stress system. Hormones (Athens) 4: 73-89.
144. Barchas J, Dacosta F, Spector S (1967) Acute pharmacology of melatonin. Nature 214: 919-920. DOI: https://doi.org/10.1038/214919a0.
145. Erland LA, Saxena PK (2017) Melatonin natural health products and supplements: presence of serotonin and significant variability of melatonin content. J. Clin. Sleep Med..13: 275-281. DOI: 10.5664/jcsm.6462.
146. Kaya O, Gokdemir K, Kilic M, Baltaci AK (2006) Melatonin supplementation to rats subjected to acute swimming exercise: Its effect on plasma lactate levels and relation with zinc. Neuro. Endocrinol. Lett. 27: 263-266.
147. Trionfante CP, Davis GR, Farney TM, Miskowiec RW, Nelson AG (2017) A pre-exercise dose of melatonin can alter substrate use during exercise. Int. J. Exerc. Sci.10: 1029.
148. Farjallah MA, Hammouda O, Ben Mahmoud L, Graja A, Ghattassi K, Boudaya M, Jammoussi K, Sahnoun Z, Souissi N (2018) Melatonin supplementation ameliorates oxidative stress, antioxidant status and physical performances recovery during a soccer training camp. Biol. Rhythm Res. 51: 441-452. DOI: https://doi.org/10.1080/09291016.2018.1533749.
149. Stratos I, Richter N, Rotter R, Li Z, Zechner D, Mittlmeier T, Vollmar B (2012) Melatonin restores muscle regeneration and enhances muscle function after crush injury in rats. J. Pineal Res. 52: 62-70. DOI: 10.1111/j.1600-079X.2011.00919.x.
150. Maarman GJ, Reiter RJ (2018) Melatonin therapy for blunt trauma and strenuous exercise: A mechanism involving cytokines, NFκB, Akt, MAFBX and MURF-1. J. Sports Sci. 36: 1897-1901. DOI: 10.1080/02640414.2018.1424491.
151. Hara M, Iigo M, Ohtani-Kaneko R, Nakamura N, Suzuki T, Reiter RJ, Hirata K (1997) Administration of melatonin and related indoles prevents exercise-induced cellular oxidative changes in rats. Neurosignals 6: 90-100. DOI: 10.1159/000109113.
152. Bicer M, Baltaci SB, Patlar S, Mogulkoc R, Baltaci AK (2018) Melatonin has a protective effect against lipid peroxidation in the bone tissue of diabetic rats subjected to acute swimming exercise. Horm. Mol. Biol. Clin. Investig. 34. DOI: 10.1515/hmbci-2017-0079.
153. Costa VM, Carvalho F, Duarte JA, Bastos MD, Remião F (2013) The heart as a target for xenobiotic toxicity: the cardiac susceptibility to oxidative stress. Chem. Res. Toxicol. 26: 1285-1311. DOI: 10.1021/tx400130v.
154. Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 301: H2181-2190. DOI: 10.1152/ajpheart.00554.2011.
155. Hallqvist J, Möller J, Ahlbom A, Diderichsen F, Reuterwall C, Faire UD (2000) Does heavy physical exertion trigger myocardial infarction? A case-crossover analysis nested in a population-based case-referent study. Am. J. Epidemiol. 151: 459-467. DOI: 10.1093/oxfordjournals.aje.a010231.
156. Rowell LB (1974) Human cardiovascular adjustments to exercise and thermal stress. Physiol. Rev. 54: 75-159. DOI: 10.1152/physrev.1974.54.1.75.
157. Kindermann W, Schnabel A, Schmitt WM, Biro G, Cassens J, Weber F (1982) Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise. Eur. J. Appl. Physiol. Occup. Physiol. 49: 389-399. DOI: 10.1007/BF00441300.
158. Brooks S, Cheetham M, Williams C (1985) Endurance training and the catecholamine response to brief maximum exercise in man. J. Physiol. 361: 81.
159. Baker JS, Buchan D (2017) Metabolic stress and high intensity exercise. Phys. Med. Rehabil. Res .2: 1-2. DOI: 10.15761/PMRR.1000136.
160. Zhang DY, Anderson AS (2014) The sympathetic nervous system and heart failure. Cardiol. Clin. 32: 33-45. doi: 10.1016/j.ccl.2013.09.010.
161. Viswanathan M, Hissa R, George JC (1986) Suppression of sympathetic nervous system by short photoperiod and melatonin in the Syrian hamster. Life Sci. 38: 73-79. DOI: 10.1016/0024-3205(86)90277-8.
162. Behonick GS, Novak MJ, Nealley EW, Baskin SI (2001) Toxicology update: the cardiotoxicity of the oxidative stress metabolites of catecholamines (aminochromes). J. Appl. Toxicol. 21: S15-22. DOI: 10.1002/jat.793.
163. Neri M, Cerretani D, Fiaschi AI, Laghi PF, Lazzerini PE, Maffione AB, Micheli L, Bruni G, Nencini C, Giorgi G, D'Errico S (2007) Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J. Cell Mol. Med. 11: 156-170. DOI: 10.1111/j.1582-4934.2007.00009.x.
164. Costa, VM, Silva R, Ferreira LM, Branco PS, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F (2007) Oxidation process of adrenaline in freshly isolated rat cardiomyocytes: formation of adrenochrome, quinoproteins, and GSH adduct. Chem. Res. Toxicol. 20: 1183-1191. DOI: 10.1021/tx7000916.
165. Rudra S, Mukherjee D, Dutta M, Ghosh AK, Dey M, Basu A, Pattari SK, Chattopadhyay A, Bandyopadhyay D (2014) Orally administered melatonin protects against adrenaline-induced oxidative stress in rat liver and heart: Involvement of antioxidant mechanism (s). J. Pharm. Res. 8: 303-320. DOI: http://jprsolutions.info/files/final-file56bff9adee9ad8.28733292.pdf.
166. Paulus WJ (2000) Cytokines and heart failure. Heart Fail. Monit. 1:50-56.
167. Gullestad L, Aukrust P (2001) The cytokine network in heart failure: pathogenetic importance and potential therapeutic targets. Heart Fail. Monit. 2: 8-13. DOI: 10.1159/000338166.
168. Belcastro AN, Arthur GD, Albisser TA, Raj DA (1996) Heart, liver, and skeletal muscle myeloperoxidase activity during exercise. J. Appl. Physiol. 80: 1331-1335. DOI: 10.1152/jappl.1996.80.4.1331.
169. Torres SH, De Sanctis JB, de Briceno LM, Hernandez N, Finol HJ (2004) Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J. Endocrinol. 181: 419-427. DOI: 10.1677/joe.0.1810419.
170. Tunon MJ, Garcia-Mediavilla MV, Sanchez-Campos S, Gonzalez-Gallego J (2009) Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr. Drug Metab. 10: 256-271. DOI: 10.2174/138920009787846369.
171. Tan DX, Manchester LC, Reiter RJ, Qi W, Kim SJ, El‐Sokkary GH (1998) Ischemia/reperfusion‐induced arrhythmias in the isolated rat heart: prevention by melatonin. J. Pineal Res. 25: 184-191. DOI: 10.1111/j.1600-079x.1998.tb00558.x.
172. Pialoux V, Mounier R, Rock E, Mazur A, Schmitt L, Richalet JP, Robach P, Coudert J, Fellmann N (2009) Effects of acute hypoxic exposure on prooxidant/antioxidant balance in elite endurance athletes. Int. J. Sports Med. 30: 87-93. DOI: 10.1055/s-0028-1103284.
173. Accattato F, Greco M, Pullano SA, Carè I, Fiorillo AS, Pujia A, Montalcini T, Foti DP, Brunetti A, Gulletta E (2017) Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS One 12: e0178900. DOI: 10.1371/journal.pone.0178900.
174. Noakes TD (2000) Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scand. J. Med. Sci. Spor. 10: 123-145. DOI: 10.1034/j.1600-0838.2000.010003123.x.
175. Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL (2003) Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J. Appl. Physiol. 95: 2510-2518. DOI: 10.1152/japplphysiol.00487.2003.
176. Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y (2004) Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr. Neurovasc. Res. 1: 411-420. DOI: 10.2174/1567202043361875.
177. Quindry J, French J, Hamilton K, Lee Y, Mehta JL, Powers S (2005) Exercise training provides cardioprotection against ischemia–reperfusion induced apoptosis in young and old animals. Exp. Gerontol. 40: 416-425. DOI: 10.1016/j.exger.2005.03.010.
178. Powers SK, Quindry JC, Kavazis AN (2008) Exercise-induced cardioprotection against myocardial ischemia–reperfusion injury. Free Radic. Biol. Med. 44: 193-201. DOI: 10.1016/j.freeradbiomed.2007.02.006.
179. Lee Y, Min K, Talbert EE, Kavazis AN, Smuder AJ, Willis WT, Powers SK (2012) Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Med. Sci. Sports Exerc. 44: 397-405. DOI: 10.1249/MSS.0b013e318231c037.
180. Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA 3rd, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F (2007) Exercise and acute cardiovascular events: placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology, American College of Sports Medicine. Circulation 115: 2358-2368. DOI: 10.1161/CIRCULATIONAHA.107.181485.
181. Lagneux C, Joyeux M, Demenge P, Ribuot C, Godin-Ribuot D (2000) Protective effects of melatonin against ischemia-reperfusion injury in the isolated rat heart. Life Sci. 66: 503-509. DOI: 10.1016/s0024-3205(99)00620-7.
182. Sahna E, Olmez E, Acet A (2002) Effects of physiological and pharmacological concentrations of melatonin on ischemia–reperfusion arrhythmias in rats: can the incidence of sudden cardiac death be reduced? J. Pineal Res. 32: 194-198.DOI: 10.1034/j.1600-079x.2002.1o853.x.
183. Mukherjee D, Roy SG, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandyopadhyay D (2010) Melatonin protects against isoproterenol‐induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res. 48: 251-262. DOI: 10.1111/j.1600-079X.2010.00749.x.
184. Yeung HM, Hung MW, Lau CF, Fung ML (2015) Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal Res. 58: 12-25. DOI: 10.1111/jpi.12190.
185. Vazan R, Ravingerova T (2015) Protective effect of melatonin against myocardial injury induced by epinephrine. J. Physiol. Biochem. 71: 43-49. DOI: 10.1007/s13105-014-0377-5.
186. Mukherjee D, Ghosh AK, Bandyopadhyay A, Basu A, Datta S, Pattari SK, Reiter RJ, Bandyopadhyay D (2012) Melatonin protects against isoproterenol‐induced alterations in cardiac mitochondrial energy‐metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 53: 166-179. DOI: 10.1111/j.1600-079x.2012.00984.x.
187. Nduhirabandi F, Maarman GJ (2018) Melatonin in heart failure: a promising therapeutic strategy? Molecules 23: 1819. DOI: 10.3390/molecules23071819.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


