Melatonin inhibits growth of B16 melanoma in C57BL/6 mice
Melatonin inhibits growth of melanoma
Abstract
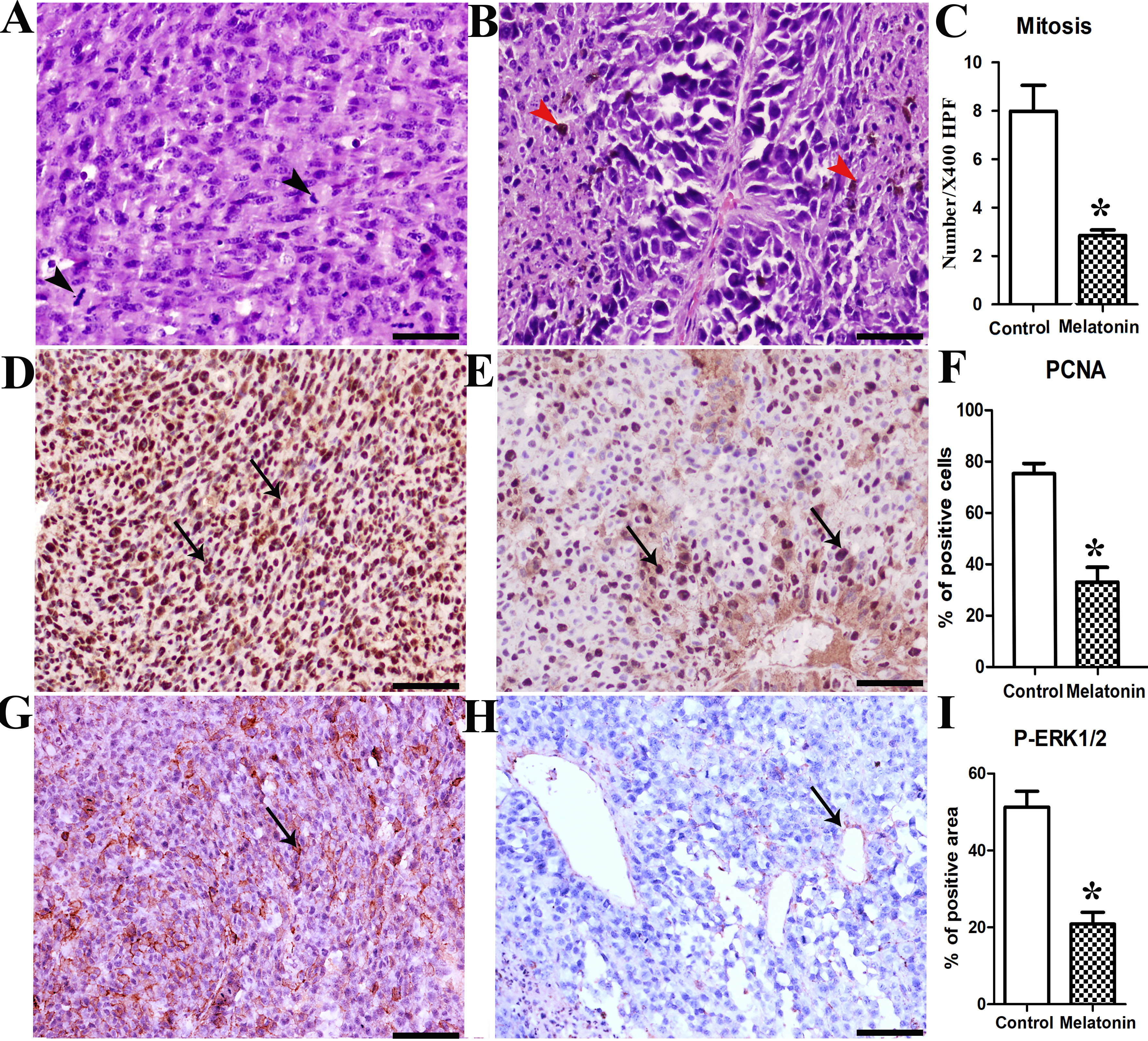
Melatonin (N-acetyl-5-methoxytryptamine) has oncostatic properties in a wide variety of tumors. In melanoma, melatonin displayed growth suppressive effects in cultured cell lines and tumors. Thus far, however, there is no evidence of orally administrated melatonin reducing melanoma tumor growth. Therefore, the current study investigated the preventive effect of melatonin on C57BL/6 mice injected with B16-F10 murine metastatic melanoma cells. The animals were divided into two groups; control (vehicle) and melatonin pre-treated with oral melatonin in the drinking water (10 mg/kg/day) for 15 days. Grossly, the control animals had a significant exponential increase in tumor size until day 33, and all control animals were dead by day 38; conversely, melatonin pre-treated mice demonstrated delayed tumour appearance as well as decreased tumour volume and increased survival rates. PCNA immunostaining corroborated these data and demonstrated a significant reduction in the number of proliferating cells in the melatonin-treated mice (P < 0.005). Interestingly, histopathological analysis revealed the presence of undifferentiated and pleomorphic cells associated with higher mitotic rate in the control group, while epithelioid-shaped cells, sometimes containing melanin were clearly identified in melatonin-treated animals. Mitochondrial parameters measurement showed greater PTP opening and increased mitochondrial nitrite level associated in melatonin-pretreated animals. Finally, the decreased P-ERK1,2 cytoplasmic expression in melatonin mice compared with the controls supports the conclusion that the MAPK signalling pathway is repressed by melatonin in B16-F10 melanoma. Collectively, these results suggest for the first time that orally-administered melatonin reduces malignant melanoma progression in vivo and increases the percent of survival by lowering tumor cells proliferation due to mitochondrial dependent cytotoxicity and decreased P-ERK1,2 expression. This study demonstrates the chemopreventive potential of melatonin against malignant melanoma in C57BL/6 mice.
References
2. Setlow RB, Woodhead AD. (1994) Temporal changes in the incidence of malignant melanoma: Explanation from action spectra. Mutat. Res. 307: 365-374.
3. Rigel DS. (2010) Epidemiology of melanoma. Semin Cutan Med Surg. 29:204-209.
4. Grabacka M, Placha W, Plonka PM, Pajak S, Urbanska K, Laidler P, et al. (2004) Inhibition of melanoma metastases by fenofibrate. Arch. Dermatol. Res. 296: 54-58.
5. Slominski A, Carlson AJ. (2014) Melanoma resistance: a bright future for academicians and a challenge for patient advocates. Mayo Clin. Proc. 89: 429-433.
6. Bonmati-Carrion MA, Alvarez-Sanchez N, Hardeland R, Madrid JA, Rol MA. (2013) A comparison of B16 melanoma cells and 3T3 fibroblasts concerning cell viability and ROS production in the presence of melatonin, tested over a wide range of concentrations. Int. J. Mol. Sci. 14: 3901-3920.
7. Acuña-Castroviejo D, Escames J, Venegas, C, Díaz-Casado ME, Lima-Cabello E, López LC, et al. (2014) Extrapineal melatonin: sources, regulation and potential functions. Cell Mol. Life Sci. 71: 2997-3025.
8. Mocayar-Marón FJ, Ferder L, Reiter RJ, Manucha W. (2020) Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J. Steroid. Biochem. Mol. Biol. 199: e105595.
9. Hardeland R, Madrid JA, Tan DX, Reiter RJ. (2012) Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 52: 139-166.
10. Tan DX, Chen L, Poeggeler B, Manchester L, Reiter RJ. (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1: 57-60.
11. Agil A, Reiter RJ, Jimenez-Aranda A, Iban-Arias R, Navarro-Alarcon M, Marchal JA, et al. (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 54:381-388.
12. Miller SC, Pandi PS, Esquifino AI, Cardinali DP, Maestroni GJ. (2006) The role of melatonin in immuno‐enhancement: potential application in cancer. Int. J. Exp. Pathol. 87: 81-87.
13. Srinivasan V, Spence DW, Trakht I, Pandi-Perumal SR, Cardinali DP, Maestroni GJ. (2008) Immunomodulation by melatonin: its significance for seasonally occurring diseases. Neuroimmunomodulation 15: 93-101.
14. Reiter RJ, Tan DX, Poeggeler B, Menendez‐Pelaez A, Chen LD, Saarela S. (1994) Melatonin as a free radical scavenger: implications for aging and age‐related diseasesa. Ann. NY. Acad. Sci.719: 1-12.
15. Gonzalez R, Sanchez A, Ferguson J, Balmer C, Daniel C, Cohn A, et al. (1991) Melatonin therapy of advanced human malignant melanoma. Melanoma Res. 1: 237-244.
16. Slominski A, Pruski D. (1993) Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp. Cell Res. 206: 189-194.
17. Panzer A, Viljoen M. (997) The validity of melatonin as an oncostatic agent. J. Pineal Res. 22: 184-202.
18. Lu KH, Lin RC, Yang JS, Yang WE, Reiter RJ, Yang SF. (2019) Molecular and cellular mechanisms of melatonin in osteosarcoma. Cells 8: e1618.
19. Reiter RJ, Rosales-Corral SA, Tan DX, Acuna -Castroviejo D, Quin D, Yang SF, Xu K. (2019) Melatonin as full service antitumor agent: inhibitionof of inititantion, progression and metastasis. Int. J. Mol. Sci. 18: E843.
20. Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, et al. (2002) Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin. Cancer Res. 8: 3728-3733.
21. Govindarajan B, Bai X, Cohen C, Zhong H, Kilroy S, Louis G, et al. (2003) Malignant transformation of melanocytes to melanoma by constitutive activation of mitogen-activated protein kinase kinase (MAPKK) signaling. J. Biol. Chem. 278: 9790-9795.
22. Wen SY, Cheng SY, Ng SC, Aneja R, Chen CJ, Huang CY, Kuo WW. (2019) Roles of p38α and p38β mitogen‑activated protein kinase isoforms in human malignant melanoma A375 cells. Int. J. Mol. Med. 44: 2123-2132.
23. Cos S, Garcia-Bolado A, Sanchez-Barcelo E. (2001) Direct antiproliferative effects of melatonin on two metastatic cell sublines of mouse melanoma (B16BL6 and PG19). Melanoma Res. 11:197-201.
24. Kadekaro AL, Andrade LN, Floeter‐Winter LM, Rollag MD, Virador V, Vieira W, et al. (2004) MT‐1 melatonin receptor expression increases the antiproliferative effect of melatonin on S‐91 murine melanoma cells. J. Pineal Res. 36:204-211.
25. Fischer T, Zmijewski M, Zbytek B, Sweatman T, Slominski R, Wortsman J, et al. (2006) Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int. J. Oncol. 29: 665-672.
26. Otalora BB, Madrid JA, Alvarez N, Vicente V, Rol MA. (2008) Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J. Pineal Res. 44: 307-315.
27. Kleszczynski K, Kim TK, Bilska B, Sarna M, Mokrzynski K, Stegemann A, et al. (2019) Melatonin exerts oncostatic capacity and decreases melanogenesis in human MNT-1 melanoma cells. J. Pineal Res.67: e12610.
28. Alvarez-Artime A, Cernuda-Cernuda R, Francisco-Artime-Naveda, Cepas V, Gonzalez-Menendez P, Fernadez-Vega S, Quiros-Gonzalez I, Sainz RM, Mayo JC. (2020) Melatonin-induced cytoskeleton reorganization leads to inhibition of melanoma cancer cell proliferation. Int. J. Mol. Sci. 21: 1-20.
29. Prados J, Melguizo C, Ortiz R, Boulaiz H, Carrillo E, Segura A, Rodríguez-Herva JJ, Ramos JL, Aránega A. (2010) Regression of established subcutaneous B16-F10 murine melanoma tumors after gef gene therapy associated with the mitochondrial apoptotic pathway. Exp. Dermatol.19: 363-371.
30. Gnaiger E, Mendez G, Hand SC. (2000) High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc. Natl. Acad. Sci. USA 97:11080-85.
31. Lopez A, Garcia JA, Escames G, Venegas C, Ortiz F, Lopez LC, et al. (2009) Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 46: 188-198.
32. Ziegler DM, Doeg K. (1962) Studies on the electron transport system. XLIII. The isolation of a succinic-coenzyme Q reductase from beef heart mitochondria. Arch. Biochem. Biophys. 97: 41-50.
33. Rieske J. (1967) Preparation and properties of reduced coenzyme Q-cytochrome c reductase (complex III of the respiratory chain). Method. Enzymol. 10: 239-245.
34. Jimenez-Aranda A, Fernandez-Vazquez G, Campos D, Tassi M, Velasco-Perez L, Tan DX, et al. (2013) Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 55: 416-423.
35. Stuehr DJ, Nathan CF. (1989) Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 169: 1543-1555.
36. Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arevalo M, et al. (2010) Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 77: 861-869.
37. Burton AL, Egger ME, Gilbert JE, Stromberg AJ, Hagendoorn L, Martin RC, et al. (2012) Assessment of mitotic rate reporting in melanoma. Am. J. Surg. 204: 969-974; discussion 974-975.
38. Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N, Werb Z. (2018) Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 20: 1349-1360.
39. Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. (1987) Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature 326: 515-517.
40. Strzalza W, Ziemienowick A. (2011) Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann. Bot. 107: 1127-1140.
41. Chen J, Wu F, Shi Y, Yang D, Xu M, Lai Y, Liu Y. (2019) Identification of key candidate genes involved in melanoma metastasis. Mol. Med. Rep. 20: 903-914.
42. Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, et al. (2015) Melatonin: an inhibitor of breast cancer. Endocr. Relat. Cancer 22: R183-204.
43. Watanabe M, Kobayashi Y, Takahashi N, Kiguchi K, Ishizuka B. (2008) Expression of melatonin receptor (MT1) and interaction between melatonin and estrogen in endometrial cancer cell line. J. Obste.t Gynaecol. Res. 34: 567-573.
44. Rodriguez-Garcia A, Mayo JC, Hevia D, Quiros-Gonzalez I, Navarro M, Sainz RM. (2012) Phenotypic changes caused by melatonin increased sensitivity of prostate cancer cells to cytokine-induced apoptosis. J. Pineal Res. 54: 33-45.
45. Proietti S, Cucina A, Reiter RJ, Bizzarri M. (2013) Molecular mechanisms of melatonin's inhibitory actions on breast cancers. Cell Mol. Life Sci. 70: 2139-2157.
46. Lu KH, Lin RC, Hsieh YH, Su SC, Reiter RJ, Yang SF. (2020) New insights into antimetastatic signaling pathways of melatonin in skeletomuscular sarcoma of childhood and adolescence. Cancer Metastasis Rev. 39: 303-320.
47. Di Bella G, Mascia F, Gualano L, Di Bella L. (2013) Melatonin anticancer effects: review. Int. J. Mo.l Sci. 14: 2410-2430.
48. Mediavilla MD, Cos S, Sanchez-Barcelo EJ. (1999) Melatonin increases p53 and p21WAF1 expression in MCF-7 human breast cancer cells in vitro. Life Sci. 65:415-420.
49. Stevens RG, Rea MS. (2001) Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control 12: 279-287.
50. Wenzel U, Nickel A, Daniel H. (2005) Melatonin potentiates flavone-induced apoptosis in human colon cancer cells by increasing the level of glycolytic end products. Int. J. Cancer. 116: 236-242.
51. Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, et al. (1998) Natural melatonin 'knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res. Mol. Brain Res. 63: 189-197.
52. Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. (2003) Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 270: 3335-3344.
53. Slominski A, Wortsman J, Tobin DJ. (2005) The cutaneous serotoninergic /melatoninergic system: securing a place under the sun. FASEB J. 19: 176-194.
54. Hill SM, Frasch T, Xiang S, Yuan L, Duplessis T, Mao L. (2009) Molecular mechanisms of melatonin anticancer effects. Integr. Cancer Ther. 8: 337-346.
55. Sarna M, Krzykawska-Serda M, Jakubowska M, Zadlo A, Urbanska K. (2019) Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci. Rep. 9: 9280.
56. Slominski A, Pisarchik A, Wortsman J. (2004a) Expression of genes coding melatonin and serotonin receptors in rodent skin. Biochim. Biophys. Acta 1680: 67-70.
57. Slominski A, Tobin DJ, Shibahara S, Wortsman J. (2004b) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 84: 1155-1228.
58. Leo S, Bianchi K, Brini M, Rizzuto R. (2005) Mitochondrial calcium signalling in cell death. FEBS J. 272:4013-4022.
59. Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. (2006) The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 273: 2077-2099.
60. Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, et al. (2006) Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 40: 553-560.
61. Giorgi C, Romagnoli A, Pinton P, Rizzuto R. (2008) Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med. 8: 119-130.
62. Orrenius S, Nicotera P, Zhivotovsky B. (2011) Cell death mechanisms and their implications in toxicology. Toxicol. Sci. 119: 3-19.
63. Johnson GL, Lapadat R. (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911-1912.
64. Sebolt-Leopold JS, Herrera R. (2004) Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer. 4: 937-947.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


