Melatonin inhibits human melanoma cells proliferation and invasion via cell cycle arrest and cytoskeleton remodeling
Oncostatic effects of melatonin in melanoma cells
Abstract
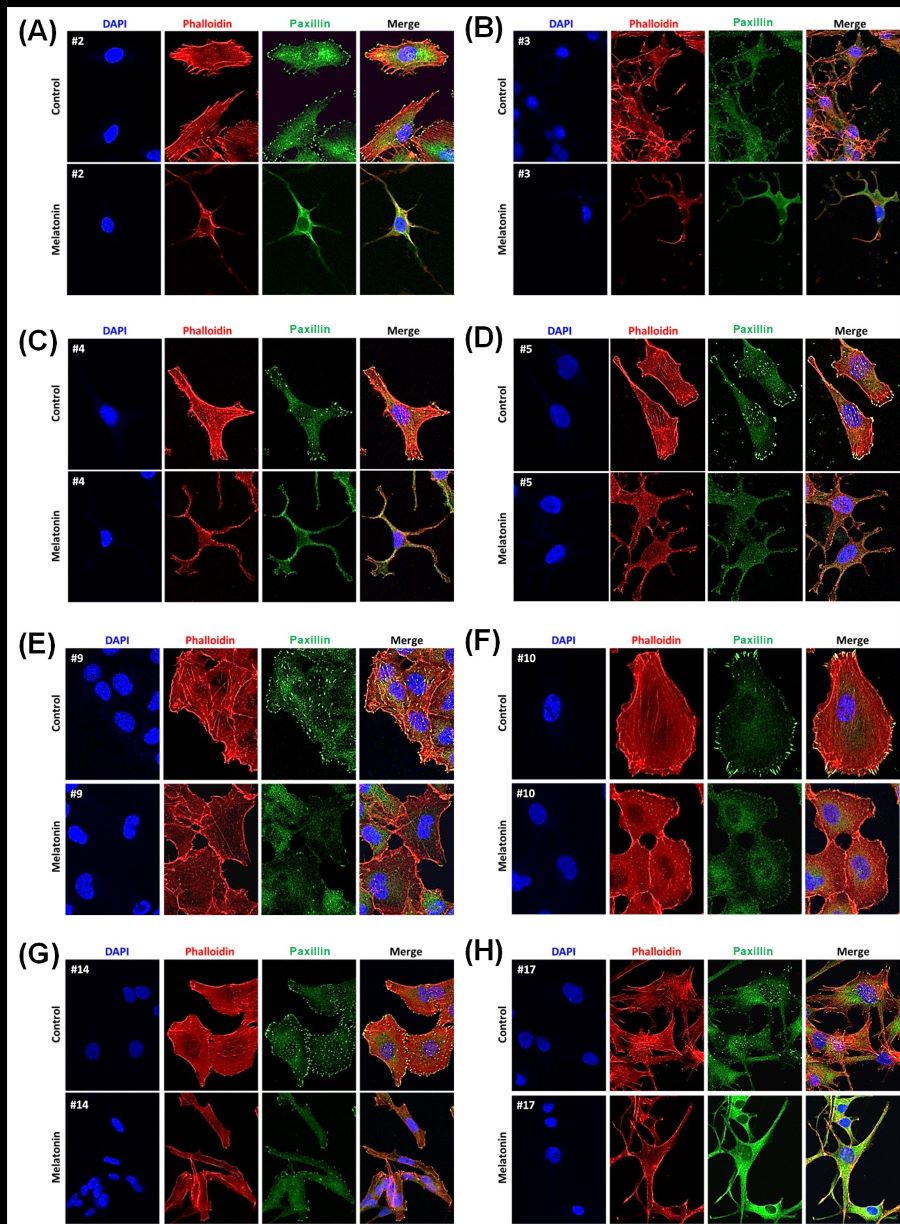
Among skin cancers, melanoma has the highest mortality rate. The heterogeneous genetic melanoma background leads to a tumor-propagating capacity particularly important in maintaining therapeutic resistance, and tumor recurrence. The identification of efficient molecules able to control melanoma progress represents an important opportunity for new therapeutic strategies, particularly in combination with the current standard-of-care treatments. In this context, several studies have reported the antitumor effects of melatonin against different types of cancer, including melanoma. Here, we describe the underlying mechanisms associated with melatonin’s activity in human melanoma cell lines, focusing on cell cycle and cytoskeleton remodeling. Interestingly, while melatonin induced melanocyte DNA replication, melanoma cells exhibited cell cycle arrest in the G1-phase. This phenomenon was associated with cyclin-D1 downregulation or p21 overexpression. The efficacy of melatonin on melanoma cells survival and proliferation was detected using the clonogenic assay, with a decrease in both the number and size of colonies. Additionally, melatonin induced a dramatic cytoskeleton remodeling in all melanoma cell lines, leading to a star-like morphology or cell swelling. The role of melatonin on melanoma cytoskeleton was associated with the actin disruption, with thinning and/or broken actin fibers, and weak and/or loss of paxillin along stress fibers. These data support the observed findings that melatonin impairs melanoma invasion in skin reconstructed models. Together, our results suggest that melatonin could be used to control melanoma growth and support basic and clinical studies on melatonin as a promising immunometabolic adjuvant for melanoma therapy.
References
2. Tripp MK, Watson M, Balk SJ, Swetter SM, Gershenwald JE (2016) State of the science on prevention and screening to reduce melanoma incidence and mortality: The time is now. CA Cancer J. Clin. 66: 460-480.
3. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics, 2016. CA. Cancer J. Clin. 66: 271-289.
4. Tormo D, Checińska A, Alonso-Curbelo D, Pérez-Guijarro E, Cañón E, Riveiro-Falkenbach E, Calvo TG, Larribere L, Megías D, Mulero F, Piris MA, Dash R, Barral PM, Rodríguez-Peralto JL, Ortiz-Romero P, Tüting T, Fisher PB, Soengas MS (2009) Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells. Cancer Cell 16: 103-114.
5. Buchbinder EI, Hodi FS (2016) Melanoma in 2015: Immune-checkpoint blockade - durable cancer control. Nat. Rev. Clin. Oncol. 13: 77-78
6. Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, Larkin J, Nyakas M, Dutriaux C, Haydon A, Robert C, Mortier L, Schachter J, Schadendorf D, Lesimple T, Plummer R, Ji R, Zhang P, Mookerjee B, Legos J, Kefford R, Dummer R, Kirkwood JM (2017) Adjuvant dabrafenib plus trametinib in stage III BRAF -mutated melanoma. N. Engl. J. Med. 377: 1813-1823.
7. Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 34: 75-78.
8. Al-Omary FAM (2013) Melatonin. comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 38: 159-226.
9. Bubenik GA (2001) Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol. Signals Recept. 10: 350-366.
10. Moreno ACR, Clara RO, Coimbra JB, Júlio AR, Albuquerque RC, Oliveira EM, Maria-Engler SS, Campa A (2013) The expanding roles of 1-methyl-tryptophan (1-MT): In addition to inhibiting kynurenine production, 1-MT activates the synthesis of melatonin in skin cells. FEBS J. 280: 4782-9472.
11. Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D (2006) The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs 7: 423-431.
12. Zimmerman S, Reiter RJ (2019) Melatonin and the optics of the human body. Melatonin Res. 2: 138-160.
13. Reiter RJ, Ma Q, Sharma R (2020) Melatonin in mitochondria: mitigating clear and present dangers. Physiology 35: 86-95.
14. Fernández A, Ordõñez R, Reiter RJ, González-Gallego J, Mauriz JL (2015) Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 59: 292-307.
15. Sagrillo-Fagundes L, Assunção Salustiano EM, Ruano R, Markus RP, Vaillancourt C (2018) Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 65: e12520.
16. Paroni R, Terraneo L, Bonomini F, Finati E, Virgili E, Bianciardi P, Favero G, Fraschini F, Reiter RJ, Rezzani R, Samaja M (2014) Antitumour activity of melatonin in a mouse model of human prostate cancer: Relationship with hypoxia signalling. J. Pineal Res. 57: 43-52.
17. Proietti S, Cucina A, Reiter RJ, Bizzarri M (2013) Molecular mechanisms of melatonin’s inhibitory actions on breast cancers. Cell. Mol. Life Sci. 70: 2139-2157.
18. Otálora BB, Madrid JA, Alvarez N, Vicente V, Rol MA (2008) Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J. Pineal Res. 44: 307-315.
19. Moreno ACR, Porchia BFMM, Pagni RL, Souza P da C, Pegoraro R, Rodrigues KB, Barros TB, Aps LRMM, de Araújo EF, Calich VLG, Ferreira LCS (2018) The Combined Use of Melatonin and an Indoleamine 2,3-Dioxygenase-1 Inhibitor Enhances Vaccine-Induced Protective Cellular Immunity to HPV16-Associated Tumors. Front. Immunol. 9: 1914.
20. Lissoni P (2007) Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol. Biol. 55: 201-204.
21. Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P (2008) Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111: 2152-2154.
22. Munn DH, Mellor AL (2007) Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 117: 1147-1154.
23. Faião-Flores F, Alves-Fernandes DK, Pennacchi PC, Sandri S, Vicente ALSA, Scapulatempo-Neto C, Vazquez VL, Reis RM, Chauhan J, Goding CR, Smalley KS, Maria-Engler SS (2017) Targeting the hedgehog transcription factors GLI1 and GLI2 restores sensitivity to vemurafenib-resistant human melanoma cells. Oncogene 36: 1849-1861.
24. Sandri S, Faião-Flores F, Tiago M, Pennacchi PC, Massaro RR, Alves-Fernandes DK, Berardinelli GN, Evangelista AF, de Lima Vazquez V, Reis RM, Maria-Engler SS (2016) Vemurafenib resistance increases melanoma invasiveness and modulates the tumor microenvironment by MMP-2 upregulation. Pharmacol. Res. 111: 523-533.
25. Pennacchi PC, de Almeida MES, Gomes OLA, Faião-Flores F, de Araújo Crepaldi MC, dos Santos MF, de Moraes Barros SB, Maria-Engler SS1 (2015) Glycated reconstructed human skin as a platform to study the pathogenesis of skin aging. Tissue Eng. Part A 21: 2417-2425.
26. Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Lawrence DP, Logan TF, Schuchter LM, Nair S, Fecher L, Buchbinder EI, Berghorn E, Ruisi M, Kong G, Jiang J, Horak C, Hodi FS (2016) Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 17: 943-955.
27. Sainz RM, Mayo JC, Tan DX, León J, Manchester L, Reiter RJ (2005) Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate 63: 29-43.
28. Martín V, Herrera F, Carrera-Gonzalez P, García-Santos G, Antolín I, Rodriguez-Blanco J, Rodriguez C (2006) Intracellular signaling pathways involved in the cell growth inhibition of glioma cells by melatonin. Cancer Res. 66: 1081-1088.
29. García-Santos G, Antolín I, Herrera F, Martín V, Rodriguez-Blanco J, del Pilar Carrera M, Rodriguez C (2006) Melatonin induces apoptosis in human neuroblastoma cancer cells. J. Pineal Res. 41: 130-135.
30. Cabrera J, Negrín G, Estévez F, Loro J, Reiter RJ, Quintana J (2010) Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J. Pineal Res. 49: 45-54.
31. Fischer TW, mijewski MA, Zbytek B, Sweatman TW, Slominski RM, Wortsman J, Slominski A (2006) Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int. J. Oncol. 29: 665-672.
32. Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G (1993) Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 7: 812-821.
33. Abbas T, Dutta A (2009) P21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 9: 400-414.
34. Kim CH, Yoo YM (2010) Melatonin induces apoptotic cell death via p53 in LNCaP cells. Korean J. Physiol. Pharmacol. 14: 365-369.
35. Cini G, Neri B, Pacini A, Cesati V, Sassoli C, Quattrone S, D'Apolito M, Fazio A, Scapagnini G, Provenzani A, Quattrone A (2005) Antiproliferative activity of melatonin by transcriptional inhibition of cyclin D1 expression: A molecular basis for melatonin-induced oncostatic effects. J. Pineal Res. 39: 12-20.
36. Souza AV, Visconti MA, Lauro Castrucci AM (2003) Melatonin biological activity and binding sites in human melanoma cells. J. Pineal Res. 34: 242-248.
37. Cos S, Garcia-Bolado A, Sánchez-Barceló EJ (2001) Direct antiproliferative effects of melatonin on two metastatic cell sublines of mouse melanoma (B16BL6 and PG19). Melanoma Res. 11: 197-201.
38. Hu DN, Roberts JE (1997) Melatonin inhibits growth of cultured human uveal melanoma cells. Melanoma Res. 7: 27-31.
39. Hu DN, McCormick SA, Roberts JE (1998) Effects of melatonin, its precursors and derivatives on the growth of cultured human uveal melanoma cells. Melanoma Res. 8: 205-210.
40. Gatti G, Lucini V, Dugnani S, Calastretti A, Spadoni G, Bedini A, Rivara S, Mor M, Canti G, Scaglione F, Bevilacqua A (2017) Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget 8: 68338-68353.
41. Cifdaloz M, Osterloh L, Graña O, Riveiro-Falkenbach E, Ximénez-Embún P, Muñoz J, Tejedo C, Calvo TG, Karras P, Olmeda D, Miñana B, Gómez-López G, Cañon E, Eyras E, Guo H, Kappes F, Ortiz-Romero PL, Rodríguez-Peralto JL, Megías D, Valcárcel J, Soengas MS (2017) Systems analysis identifies melanoma-enriched pro-oncogenic networks controlled by the RNA binding protein CELF1. Nat. Commun. 8: 2249.
42. Benot S, Goberna R, Reiter RJ, Garcia-Mauriño S, Osuna C, Guerrero JM (1999) Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J. Pineal Res. 27: 59-64.
43. Hevia D, Mayo JC, Quiros I, Gomez-Cordoves C, Sainz RM (2010) Monitoring intracellular melatonin levels in human prostate normal and cancer cells by HPLC. Anal Bioanal. Chem. 397: 1235-1244.
44. Martins E, Ferreira ACF, Skorupa AL, Afeche SC, Cipolla-Neto J, Costa Rosa LFBP (2004) Tryptophan consumption and indoleamines production by peritoneal cavity macrophages. J. Leukoc. Biol. 75: 1116-1121.
45. Carrillo-Vico A, Lardone PJ, Fernández-Santos JM, Martín-Lacave I, Calvo JR, Karasek M, Guerrero JM (2005) Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J. Clin. Endocrinol. Metab. 90: 992-1000.
46. Pontes GN, Cardoso EC, Carneiro-Sampaio MMS, Markus RP (2006) Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes) - Melatonin in human colostrum and colostrum phagocytes. J. Pineal Res. 41: 136-141.
47. Carrillo-Vico A, Calvo JR, Abreu P, Lardone PJ, García-Mauriño S, Reiter RJ, Guerrero JM (2004) Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 18: 537-539.
48. Matsui DH, Machado-Santelli GM (1997) Alterations in F-actin distribution in cells treated with melatonin. J. Pineal Res. 23: 169-175.
49. Carragher NO, Frame MC (2004) Focal adhesion and actin dynamics: A place where kinases and proteases meet to promote invasion. Trends Cell Biol. 14: 241-249.
50. Ortíz-López L, Morales-Mulia S, Ramírez-Rodríguez G, Benítez-King G (2009) ROCK-regulated cytoskeletal dynamics participate in the inhibitory effect of melatonin on cancer cell migration. J. Pineal Res. 46: 15-21.
51. Witt-Enderby PA, MacKenzie RS, McKeon RM, Carroll EA, Bordt SL, Melan MA (2000) Melatonin induction of filamentous structures in non-neuronal cells that is dependent on expression of the human mt1 melatonin receptor. Cell Motil. Cytoskeleton 46: 28-42.
52. Benítez‐King G, Huerto‐Delgadillo L, Antón‐Tay F (1990) Melatonin Effects on the cytoskeletal organization of MDCK and neuroblastoma N1E‐115 cells. J. Pineal Res. 9: 209-220.
53. Chen DL, Wang ZQ, Ren C, Zeng ZL, Wang DS, Luo HY, Wang F, Qiu MZ, Bai L, Zhang DS, Wang FH, Li YH, Xu RH (2013) Abnormal expression of paxillin correlates with tumor progression and poor survival in patients with gastric cancer. J. Transl. Med. 11: 277.
54. Jagadeeswaran R, Surawska H, Krishnaswamy S, Janamanchi V, Mackinnon AC, Seiwert TY, Loganathan S, Kanteti R, Reichman T, Nallasura V, Schwartz S, Faoro L, Wang YC, Girard L, Tretiakova MS, Ahmed S, Zumba O, Soulii L, Bindokas VP, Szeto LL, Gordon GJ, Bueno R, Sugarbaker D, Lingen MW, Sattler M, Krausz T, Vigneswaran W, Natarajan V, Minna J, Vokes EE, Ferguson MK, Husain AN, Salgia R (2008) Paxillin is a target for somatic mutations in lung cancer: Implications for cell growth and invasion. Cancer Res. 68: 132-142.
55. Li HG, Xie DR, Shen XM, Li HH, Zeng H, Zeng YJ (2005) Clinicopathological significance of expression of paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World J. Gastroenterol. 11: 1445-1451.
56. Sen A, O’Malley K, Wang Z, Raj G V., DeFranco DB, Hammes SR (2010) Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J. Biol. Chem. 285: 28787-28795.
57. German AE, Mammoto T, Jiang E, Ingber DE, Mammoto A (2014) Paxillin controls endothelial cell migration and tumor angiogenesis by altering neuropilin 2 expression. J. Cell Sci. 127: 1672-1683.
58. Velasco-Velázquez MA, Salinas-Jazmín N, Mendoza-Patiño N, Mandoki JJ (2008) Reduced paxillin expression contributes to the antimetastatic effect of 4-hydroxycoumarin on B16-F10 melanoma cells. Cancer Cell Int. 8: 8.
59. Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J (2003) Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell Physiol. 196: 144-153.
60. Roberts JE, F. Wiechmann A, Hu D-N (2000) Melatonin receptors in human uveal melanocytes and melanoma cells. J. Pineal Res. 28: 165-171.
61. Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, Jing C, Johansson O (2002) Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 16: 896-898.
62. Pourhanifeh MH, Mahdavinia M, Reiter RJ, Asemi Z (2019) Potential use of melatonin in skin cancer treatment: A review of current biological evidence. J. Cell. Physiol. 234: 12142-12148.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


