Melatonin chelates iron and binds directly with phenylhydrazine to provide protection against phenylhydrazine induced oxidative damage in red blood cells along with its antioxidant mechanisms: an in vitro study
Antioxidant mechanism of melatonin in RBC
Abstract
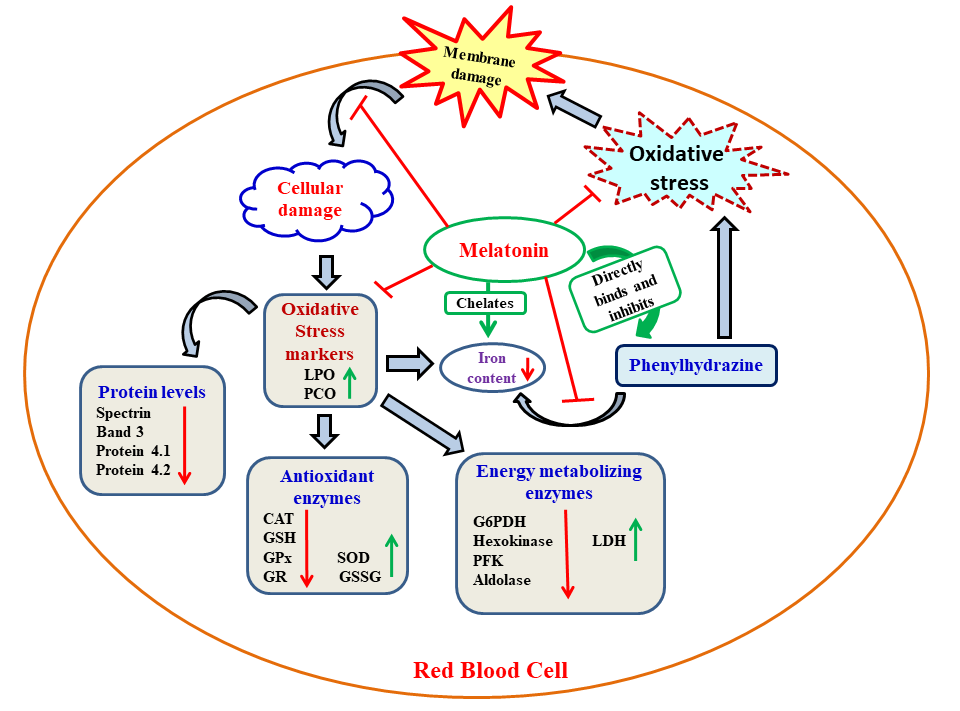
Oxidative stress is an important causative factor for a number of diseases. Phenylhydrazine (PHZ) is a widely accepted model for studying hemolytic anemia by induction of oxidative stress. In the present study, goat red blood cells (RBCs) were incubated in vitro with PHZ (1mM) to generate oxidative stress. To test whether melatonin exhibits protective effects on PHZ induced RBC damage and to explore its potential molecular mechanisms, different concentrations of melatonin (5, 10, 20 and 40 nmoles/ml) were also included. PHZ caused altered profiles on biomarkers of oxidative stress and antioxidative as well as glucose metabolic enzymes in RBCs. These alterations indicated a development of oxidative stress. Melatonin at a concentration of 40 nmoles/ml provided optimal protection against all alterations induced by PHZ. The important cellular membrane proteins, including spectrin and actin, were also damaged by PHZ and this led to RBC deformation similar to that of observed in severe β-thalassaemia; the RBC deformation was also prevented by melatonin. Binding profiles of melatonin with PHZ and ferrous iron indicated favorable binding of melatonin with both of them, respectively. Thus, in addition to the direct antioxidant and free radical scavenging capability, melatonin also inhibited iron overloading by chelating iron and binding with the PHZ. This action of melatonin further reduces free radical generation. Based on the results, melatonin may provide therapeutic relevance to ß-thalassemia and other hemolytic RBC disorders involving oxidative stress.
References
https://www.journals.elsevier.com/bba-general-subjects.
2. Winterboun CC (1985) Free radical production and oxidative reactions of hemoglobin. Environ. Health Perspect. 64: 321-330. https://ehp.niehs.nih.gov/.
3. Vilsen B, Nielsen H (1984) Reaction of phenylhydrazine with erythrocytes. Clin. Pharmaco. l33: 2739-2748.https://www.clinicalpharmacology.com/
4. Ferrali M, Signorini C, Sugherini L, Pompella A, Lodovici M, Caciotti B, Ciccoli L, Comporti M (1997) Release of free, redox-active iron in the liver and DNA oxidative damage following phenylhydrazineintoxication. Biochem. Pharmacol. 53: 1743–1751. https://www.journals.elsevier.com/biochemical-pharmacology.5. Paul S, Ghosh D, Ghosh AK, Bhowmick D, Bandyopadhyay D, Chattopadhyay A (2016) Aqueous bark extract of Terminalia arjuna protects against phenylhydrazine induced oxidative damage in goat red blood cell membrane bound and metabolic enzymes. Int. J. Pharm. and Pharmaceutical Sciences 8 (5): 62-70. https://innovareacademics.in/journals/index.php/ijpps.
6. Schrier SL, Mohandas N (1992) Globin-chain specificity of oxidation- induced changes in red blood cell membrane properties. Blood 79: 1586–1592. www.bloodjournal.org/.
7. Tan DX, Chen LD, Poeggeler B, Manchester L C, Reiter R J (1993) Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocrine J. 1: 57–60. https://www.jstage.jst.go.jp/browse/endocrj.
8. Reiter RJ, Tan DX, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress: A review. J. Biomed. Sci. 7: 444–458. https://jbiomedsci.biomedcentral.com/
9. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36: 1–9. https://onlinelibrary.wiley.com/journal/1600079x.
10. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253-278. https://www.ncbi.nlm.nih.gov/pubmed/27500468
https://onlinelibrary.wiley.com/journal/1600079x.
11.Galano A, Reiter R J (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 65: 1-33. e12514. doi: 10.1111/jpi.12514 https://onlinelibrary.wiley.com/journal/1600079x.
12. Arduini A, Storto S, Belfiglio M, Belfiglio M, Sourti R, Federici G (1989) Mechanism of spectrin degradation induced by phenylhydrazine in intact human erythrocytes. Biochim. et. Biophys. Acta 979: 1-6. https://www.journals.elsevier.com/bba-general-subjects.
13. Buege JA, Aust SG (1978) Microsomal Lipid Peroxidation. Meth. Enzymol. 52: 302–310. https://www.sciencedirect.com/bookseries/methods-in-enzymology.
14. Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 25: 192-205. https://www.sciencedirect.com/journal/analytical-biochemistry.
15. Levine RL, Williams JA, Stadtman ER, et al. (1994) Carbonyl assays for determination of oxidatively modified proteins. Meth. Enzymol. 233: 346-357. https://www.sciencedirect.com/bookseries/methods-in-enzymology.
16. Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyragallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47: 469-474. https://onlinelibrary.wiley.com/toc/17424658/271/1.
17. McChord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244: 6049-6055. www.jbc.org/.
18. Beers Jr, RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 95: 133-140. www.jbc.org/.
19. Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70:158-169. https://www.sciencedirect.com/journal/journal-of-laboratory-and-clinical-medicine.
20. Krohne-Ehrich G, Schirmer RH, Untucht-Grau R (1977) Glutathione reductase from human erythrocytes. Isolation of the enzyme and sequence analysis of the redox-active peptide. Eur. J. Biochem. 80: 65-71. https://onlinelibrary.wiley.com/toc/17424658/271/1.
21. Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione -S- transferases, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249: 7130-7139. www.jbc.org/.
22. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. https://www.nature.com/.
23. Beutlet E (1989) Glucose 6 phosphate dehydrogenase-New Perspectives. Blood 73: 1397-1401. www.bloodjournal.org/.
24. Haritos A, Rosenmeyer M (1986) Purification and physical properties of Hexokinase from human erythrocytes. Biochem. et Biophysics. Acta 873: 335-339. https://www.elsevier.com/life-sciences/bba.
25. Layzer RBT, Rowland LP, Bank WJ (1969) Physical and Kinetic Properties of Human Phosphofructokinase from Skeletal Muscle and Erythrocytes. J. Biol. Chem. 244: 3823-3831. www.jbc.org/.
26. Castillo J, Calveras J, Casas J, Mitjans M et al. (2006) Fructose-6-Phosphate Aldolase in Organic Synthesis: Preparation of D-Fagomine, N-Alkylated Derivatives, and Preliminary Biological Assays. Org. Lett. 8: 6067-6070. https://pubs.acs.org/journal/orlef7.
27. Marar T, Singh K, Bhori M, Dhanesha M (2015) Impact of antioxidant supplementation on toxicity of methotrexate: an in vitro study on erythrocytes using vitamin E. Asian J. Pharm. Clin. Res. 8: 339-343. https://innovareacademics.in/journals/index.
php/ajpcr
28. Ajila CM, Prasada Rao UJS (2007) Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangiferaindica L. peel extract. Food Chem. Toxicol. 46: 303-309. https://www.journals.elsevier.com/food-and-chemical-toxicology.
29. Mishra R, Sarkar D, Bhattacharya S, Mallik S, Chakraborty M, Mukherjee D, Kar M, Mishra R (2013) Quantifying morphological alteration of RBC population from light scattering data. Clin. Hemorheol. Microcirc. 59: 287-300. https://www.iospress.nl/journal/clinical-hemorheology-and-microcirculation/.30. Rajurkar NS, Patil SF, Zatakia NH (2012) Assessment of iron and haemoglobin status in working women of various age groups. J. Chem. Pharma. Re.s 4: 2300-2305. www.jocpr.com/.
31. Mitra E, Ghosh AK, Ghosh D, Mukherjee D, Chattopadhyay A, Dutta S, Pattari S K, Bandyopadhyay D. (2012) Protective effect of aqueous curry leaf (Murrayakoenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 50: 1340-53. https://www.journals.elsevier.com/food-and-chemical-toxicology.
32. Rajrathnam K., Rosgen J. (2014) Isothermal titration calorimetry of membrane proteins — Progress and challenges. Biochim.et Biophys. Acta 1838: 69–77. https://www.elsevier.com/life-sciences/bba.
33. Lowry OH, Rosebrough NJ, Farr AL, Randall R J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265-275. www.jbc.org/.
34. Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72: 248-254. https://www.sciencedirect.com/journal/analytical-biochemistry.
35. Kuchino Y, Mori F, Kasa H (1987) Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature 327: 77-79. https://www.nature.com/
36. Esterbauer H, Schaur RJ, Zolner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11: 81-128. https://www.journals.elsevier.com/free-radical-biology-and-medicine.
37. Ferrali M, Signorini C, Sugherini L (1997) Release of free, redox-active iron in the liver and DNA oxidative damage following phenylhydrazine intoxication. Biochem. Pharmacol. 3: 1743–1751. https://www.journals.elsevier.com/biochemical-pharmacology
38. Ferrali M, Signorini C, Ciccoli L (1992) Iron release and membrane damage in erythrocytes exposed to oxidizing agents, phenylhydrazine, divicine and isouramil. Biochem. J. 285:295–301. www.biochemj.org/.
39. ManchesterL C,Coto-Montes A, Boga JA (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59: 403-419. https://onlinelibrary.wiley.com/journal/1600079x.
40. Rodriguez C, Mayo JC, Sainz RM (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36: 1-9. https://onlinelibrary.wiley.com/journal/1600079x.
41. Tomas-Zapico C, Coto-Montes A (2005) A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 39: 99-104. https://onlinelibrary.wiley.com/journal/1600079x.
42. Sewerynek E, Melchiorri D, Chen L (1995) Melatonin reduces basal and bacterial lipopolysaccharide-induced lipid peroxidation in vitro. Free Radic. Biol. Med. 19: 993-909. https://www.journals.elsevier.com/free-radical-biology-and-medicine.
43. Hu W, Ma Z, Jiang S (2016) Melatonin: the dawning of a treatment for fibrosis? J. Pineal Res. 60: 121-131. https://onlinelibrary.wiley.com/journal/1600079x.
44. Bashan N, Pothashnik R, Feozer R (1982) The effect of oxidative agents on normal and G6PD deficient red blood cell membranes, in Weatheral DJ, Fiorelly G, Gorini S (eds): Advances in Red Cell Biology New York, NY, Raven, p 365.
45. Flynn TP, Johnson GJ, Allen DW (1981) Mechanism of decreased erythrocyte deformability and survival in glucose 6 phosphate dehydrogenase mutants, in Alan R, Liss A (eds): Recent Clinical and Experimental Advances New York, NY, Raven, p 231.
46. Johnson GJ, Allen DW, Cadman S, Fairbank V S, White J G, Lampkin B C, Kaplan M E (1979) Red-cell-membrane polypeptides aggregates in glucose-6-phosphate dehydrogenase mutants with chronic hemolytic disease. N. Engl. J. Med. 301: 522–527. https://www.nejm.org/
47. Shukla P, Yadav KN, Singh P, Bansode F W, Singh RK (2012) Phenylhydrazine induced toxicity: a review on its haematotoxicity. Inter. J. Basic. Applied. Med. Sci. 2:86–91. www.ijabmr.org/contributors.asp.
48. Kar S, Palit S, Ball WB, Das PK (2012) Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis 17: 735–747. https://link.springer.com/journal/10495
49. Chattopadhyay A, Biswas S, Bandyopadhyay D, Sarkar C, Dutta AG (2003) Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol. Cell. Biochem. 245: 43 -49 https://doi.org/10.1023/A:1022808224917; https://link.springer.com/journal/11010
50. Das N, Das Chowdhury T, Chattopadhyay A, Dutta AG (2004) Attenuation of oxidative stress induced changes in thalassemic erythrocytes by Vitamin E. Pol. J. Pharmacol. 56: 85 -96. https://www.researchgate.net/journal/1230-6002_Polish_journal_of_pharmacology.
51. Bandyopadhyay D, Bandyopadhya A, Das PK, Reiter R J (2002) Melatonin protects against gastric ulceration and increases the efficacy of ranitidine and omeprazole in reducing gastric damage. J. Pineal. Res. 33: 1-7. https://onlinelibrary.wiley.com/journal/1600079x.
52. Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Rodriguez C (2002) Melatonin regulation of antioxidant enzyme gene expression. Cell. Mol. Life Sci. 59: 1706–1713. https://link.springer.com/journal/18.
53. Weaver DC, Pasternack GR, Marchesi VT (1984) The structural basis of ankyrin function. II. Identification of two functional domains. J. Biol. Chem. 259:.6170-6175. www.jbc.org.
54. Tyler JM, Hargeaves WR, Branton D (1979) Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc. Nat.l Acad. Sci. USA. 76: 5192 – 5196. http://www.pnas.org/.
55. Ungewickell E, Bennett PM, Calvert R, Ohanian V, Gratzer WV (1979) In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte Nature 280: 811-814. http://www.nature.com/.
56. Cohen CM, Foley SF (1982) The role of band 4.1 in the association of actin with erythrocyte membranes. Biochim. et Biophys. Acta. 688: 691-701. https://www.sciencedirect.com/journal/biochimica-et-biophysica-acta.
57. Morrow JS, Speicher DW, Knowles WJ, Hsu CJ, Marchesi VT (1980) Identification of functional domains of human erythrocyte spectrin. Proc. Natl. Acad. Scie. USA. 77: 6592-6596. http://www.pnas.org/.
58. Vilsen B, Nielsen H (1984) Reaction of Phenylhydrazine with erythrocytes: Cross linking of spectrin by disulfide exchange with oxidized haemoglobin. Biochem. Pharmacol. 33: 2739-2748. https://www.journals.elsevier.com/biochemical-pharmacology.
59. Galano A, Medina ME, Tan DX, Reiter RJ. (2015) Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis.
J. Pineal Res. 58:107-116. https://onlinelibrary.wiley.com/journal/1600079x.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


