Melatonin protects against cardiac damage induced by a combination of high fat diet and isoproterenol exacerbated oxidative stress in male Wistar rats
Protective role of melatonin against cardiac injury induced by oxidative stress.
Abstract
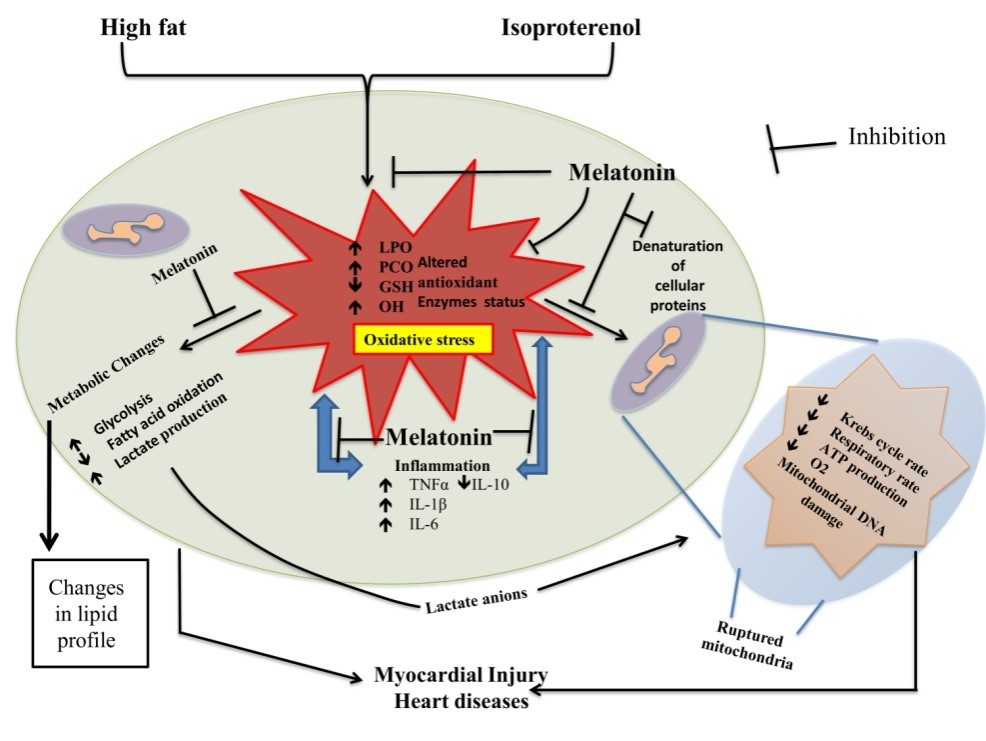
In the current study, it was found that high fat diet (60% of total kCal) (H) or/and isoproterenol (I) exacerbated oxidative stress and caused myocardial damage. This was indicated by increased levels of LPO, PCO, abnormal mitochondria and altered activities of metabolic as well as antioxidant enzymes in myocardium of rats. Melatonin at different doses (10, 20 and 40 mg/kg) effectively protected against myocardial damage induced by H or/and I and preserved all of these altered parameters. Morphological analyses showed that combination of H and I treatment led to the extensive myofibril disintegration and neutrophil infiltration. Melatonin at the dose of 40 mg/kg almost completely prevented these pathological alterations. The mechanistical studies have uncovered that the protective effects of melatonin on the myocardial damage induced by H and I are attributed to its direct and indirect antioxidative capacity, i.e., it directly scavenges free radicals and also regulates the gene expression of antioxidant enzymes. Collectively, based on the evidences gathered from the current study, it will not be unwise to suggest that melatonin can serve as an ideal therapeutic agent for those cardiovascular diseases caused by oxidative stress.
References
2. Meng X, et al. (2017) Dietary sources and bioactivities of melatonin. Nutrients 9 (4): 367. DOI: https://doi.org/10.3390/nu9040367.
3. Chowdhury I, Sengupta A, Maitra SK (2008) Melatonin: fifty years of scientific journey from the discovery in bovine pineal gland to delineation of functions in human. Indian J. Biochem. Biophys. 45 (5): 289–304.DOI: https://doi.org/123456789/2370.
4. Paul S, Naaz S, Ghosh AK, Mishra S Melatonin chelates iron and binds directly with phenylhydrazine to provide protection against phenylhydrazine induced oxidative damage in red blood cells along with its antioxidant mechanisms : an in vitro study. Melatonin Research 1 (1): 1–20. DOI: https://doi.org/10.32794/mr11250001.
5. Kumar P, Bhattacharjee B (2018) Adrenaline induced disruption of endogenous melatonergic system , antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat : an in vitro study. Melatonin Research 1 (1): 109–131. DOI: https://doi.org/10.32794/mr11250007.
6. Beyer CE, Steketee JD, Saphier D (1998) Antioxidant properties of melatonin--an emerging mystery. Biochem. Pharmacol. 56 (10): 1265–72. DOI: https://doi.org/10.1016/S0006-2952(98)00180-4.
7. Kotler M, Rodríguez C, Sáinz RM, Antolín I, Menéndez-Peláez A (1998) Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J. Pineal Res. 24 (2): 83–89. DOI: https://doi.org/10.1111/j.1600-079X.1998.tb00371.x.
8. Tan D-X, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42 (1):28–42. DOI: https://doi.org/10.1111/j.1600-079X.2006.00407.x.
9. Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 65 (1):1–33. DOI: https://doi.org/10.1111/jpi.12514.
10. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J. 5 (1):9–19. DOI: https://doi.org/10.1097/WOX.0b013e3182439613
11. Tabak O, et al. (2011) Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus. Clin. Invest. Med. 34 (3): E163-71. DOI: https://doi.org/10.25011/cim.v34i3.15189.
12. Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F (1994) Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci. 55 (15): PL271-6. DOI: https://doi.org/10.1016/0024-3205(94)00666-0.
13. Kim GH, Kim JE, Rhie SJ, Yoon S (2015) The Role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 24 (4): 325–40. DOI: https://doi.org/10.5607/en.2015.24.4.325.
14. Cervantes Gracia K, Llanas-Cornejo D, Husi H (2017) CVD and oxidative stress. J. Clin. Med. 6 (2): doi:10.3390/jcm6020022.DOI: https://doi.org/10.3390/jcm6020022.
15. Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ. Res. 107 (9): 1058–1070. DOI: https://doi org /10.1161/CIRCRESAHA.110.223545.
16. Vichova T, Motovska Z (2013) Oxidative stress: Predictive marker for coronary artery disease. Exp. Clin. Cardiol. 18 (2): e88-91.
17. Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87 (10): 840–844. DOI: https://org.doi/10.1161/01.RES.87.10.840.
18. Tsai WC, Li YH, Lin CC, Chao TH, Chen JH (2004) Effects of oxidative stress on endothelial function after a high-fat meal. Clin. Sci. 106 (3): 315–319. DOI: 10.1042/CS20030227.
19. Tawfik MK, El-Kherbetawy MK, Makary S (2018) Cardioprotective and anti-aggregatory effects of levosimendan on isoproterenol-induced myocardial injury in high-fat-fed rats involves modulation of PI3K/Akt/mTOR signaling pathway and inhibition of apoptosis. J Cardiovasc. Pharmacol. Ther. 23 (5): 456–471. DOI: https://doi.org/10.1177/1074248418763957.
20. Chang G-R, Chen W-K, Hou P-H, Mao FC (2017) Isoproterenol exacerbates hyperglycemia and modulates chromium distribution in mice fed with a high fat diet. J. Trace Elem. Med. Biol. 44: 315–321. DOI: https://doi.org/10.1016/j.jtemb.2017.09.009.
21. Dodge HT, Lord JD, Sandler H (1960) Cardiovascular effects of isoproterenol in normal subjects and subjects with congestive heart failure. Am. Heart J. 60 (1): 94–105. DOI: https://doi.org/10.1016/0002-8703(60)90063-6.
22. Khalil MI, et al. (2015) Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. Biomed. Res. Int. 2015: 1–10. DOI: http://dx.doi.org/10.1155/2015/624159.
23. Mukherjee D, et al. (2010) Melatonin protects against isoproterenol-induced myocardial injury in the rat: Antioxidative mechanisms. J. Pineal Res. 48 (3): 251–262. DOI: https://doi.org/10.1111/j.1600-079X.2010.00749.x.
24. Wang C-Y, Liao JK (2012) A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 821: 421.
25. Morris, H.P. Palmer, L.S. and Kennedy C (1933) fundamental food requirements for the growth of the rat: an experimental study of inheritance as a factor influencing food utilization in the rat. VII (University Farm, Minnesota).
26. Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28 (1): 56–63. DOI: https://doi.org/10.1093/ajcp/28.1.56.
27. Strittmatter CF (1965) Studies on avian xanthine dehydrogenases, properties and patterns of appearance during development. J. Biol. Chem. 240: 2557–2564.
28. Varcoe JS (2001) Clinical Biochemistry: Techniques and Instrumentation-A practical approach (World Scientific Publishing Company). First. DOI: https://doi.org/10.1142/4635.
29. Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol. 52: 302–310. DOI: https://doi.org/10.1016/S0076-6879(78)52032-6.
30. Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36 (3):195–203. DOI: https://doi.org/10.1111/j.1600-079X.2004.00118.x.
31. Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 25 (1): 192–205. DOI: https://doi.org/10.1016/0003-2697(68)90092-4.
32. Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 233: 346–357. DOI: https://doi.org/10.1016/S0076-6879(94)33040-9.
33. Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47 (3): 469–474. DOI: https://doi.org/10.1111/j.1432-1033.1974.tb03714.x.
34. Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195 (1): 133–140.
35. Chattopadhyay A, Biswas S, Bandyopadhyay D, Sarkar C, Datta AG (2003) Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol. Cell Biochem. 245 (1–2): 43–49. DOI: https://doi.org/10.1023/A:1022808224917.
36. Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70 (1): 158–169.
37. Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG (2000) Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem. Pharmacol. 59 (4): 419–425. DOI: https://doi.org/10.1016/S0006-2952(99)00277-4.
38. Krohne-Ehrich G, Schirmer RH, Untucht-Grau R (1977) Glutathione reductase from human erythrocytes. Isolation of the enzyme and sequence analysis of the redox-active peptide. Eur. J. Biochem. 80 (1): 65–71.
39. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 (5259): 680–5. DOI: https://www.nature.com/articles/227680a0.
40. Greenlee L, Handler P (1964) Xanthine oxidase. VI. influence of pH on substrate specificty. J. Biol. Chem. 239: 1090–1095.
41. Mitra E, et al. (2012) Protective effect of aqueous Curry leaf (Murraya koenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 50 (5): 1340–1353. DOI: https://doi.org/10.1016/j.fct.2012.01.048.
42. Dousset N, Ferretti G, Taus M, Valdiguiè P, Curatola G (1994) [49] Fluorescence analysis of lipoprotein peroxidation. Methods Enzymol. 233: 459–469. DOI: https://doi.org/10.1016/S0076-6879(94)33052-2.
43. Giulivi C, Davies KJ (1994) Dityrosine: a marker for oxidatively modified proteins and selective proteolysis. Methods Enzymol. 233: 363–371. DOI: https://doi.org/10.1016/S0076-6879(94)33042-5.
44. Layzer RB, Rowland LP, Bank WJ (1969) Physical and kinetic properties of human phosphofructokinase from skeletal muscle and erythrocytes. J. Biol. Chem. 244 (14):3823–3831.
45. Abdel-Hamid N, Ramadan M, Amgad S (2013) Glycoregulatory Enzymes as Early Diagnostic Markers during Premalignant Stage in Hepatocellular Carcinoma. Am. J. Cancer Prev. 1 (2): 14–19.
46. Noltmann EA, Gubler CJ, Kuby SA (1961) Glucose 6-phosphate dehydrogenase I. Isolation of the crystalline enzyme from yeast. J. Biol. Chem. 236: 1225–1230.
47. Chretien D, et al. (1995) An improved spectrophotometric assay of pyruvate dehydrogenase in lactate dehydrogenase contaminated mitochondrial preparations from human skeletal muscle. Clin. Chim. Acta 240 (2): 129–136. DOI: https://doi.org/10.1016/0009-8981(95)06145-6.
48. Parvin R (1969) [3] Citrate synthase from yeast: [EC 4.1.3.7 Citrate oxaloacetage-lyase (CoA-acetylating)]. Methods Enzymol. 13: 16–19. DOI: https://doi.org/10.1016/0076-6879(69)13007-4.
49. Duncan MJ, Fraenkel DG (1979) alpha-Ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J. Bacteriol. 137 (1): 415–419. https://jb.asm.org/content/137/1/415.
50. Veeger C, DerVartanian DV, Zeylemaker WP (1969) [16] Succinate dehydrogenase: [EC 1.3.99.1 Succinate: (acceptor) oxidoreductase]. Methods Enzymol. 13: 81–90. DOI: https://doi.org/10.1016/0076-6879(69)13020-7.
51. Goyal N, Srivastava VM (1995) Oxidation and reduction of cytochrome c by mitochondrial enzymes of Setaria cervi. J. Helminthol. 69 (1): 13–17. DOI: https://doi.org/10.1017/S0022149X00013778.
52. Middleton B (1975) [17] 3-Ketoacyl-CoA thiolases of mammalian tissues. Methods Enzymol. 35: 128–136. DOI: https://doi.org/10.1016/0076-6879(75)35148-3.
53. Delafield FP, Doudoroff M (1969) β-Hydroxybutyrate dehydrogenase from Pseudomonas lemoignei: EC 1.1.1.30 d-3-Hydroxybutyrate: NAD oxidoreductase. Methods Enzymol. 14: 227–231. DOI: https://doi.org/10.1016/S0076-6879(69)14044-6.
54. Wroblewski F, Ladue JS (1955) Lactic Dehydrogenase Activity in Blood. Exp. Biol. Med. 90 (1): 210–213. DOI: 10.3181/00379727-90-21985.
55. Šimaga Š, Abramic M, Osmak M, Babik D, Ilic-Forko J (2008) Total tissue lactate dehydrogenase activity in endometrial carcinoma. Int. J. Gynecol. Cancer. 18 (6): 1272–1278. DOI: 10.1111/j.1525-1438.2008.01196.x.
56. Bancroft, John D and Gamble M (2008) Theory and practice of histological techniques (Elsevier Health Sciences, London). Sixth.
57. Noronha-Dutra AA, Steen EM, Woolf N (1984) The early changes induced by isoproterenol in the endocardium and adjacent myocardium. Am. J. Pathol. 114.(2):231–239.
58. Mukherjee D, et al. (2012) Melatonin protects against isoproterenol-induced alterations in cardiac mitochondrial energy-metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 53 (2): 166–179. DOI: https://doi.org/10.1111/j.1600-079X.2012.00984.x.
59. Mukherjee D, et al. (2013) Beneficial role of melatonin in the complete recovery from isoproterenol-induced cardiac injury in rats. Int. J. Pharm. Pharm. Sci. 5 (2):
60. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193 (1): 265–275.
61. Ternacle J, et al. (2017) Short-term high-fat diet compromises myocardial function: a radial strain rate imaging study. Eur. Hear J. Cardiovasc. Imaging. 18 (11): 1283–1291. DOI: https://doi.org/10.1093/ehjci/jew316.
62. Benson TW, et al. (2018) A single high-fat meal provokes pathological erythrocyte remodeling and increases myeloperoxidase levels: implications for acute coronary syndrome. Lab. Investig. 98 (10): 1300–1310.https://www.nature.com/articles/s41374-018-0038-3
63. Sagan D, Stepniak J, Gesing A, Lewinski A, Karbownik-Lewinska M (2017) Melatonin reverses the enhanced oxidative damage to membrane lipids and improves skin biophysical characteristics in former-smokers - A study in postmenopausal women. Ann. Agric. Environ. Med. 24 (4): 659–666. DOI: 10.5604/12321966.1235174.
64. Venditti P, Masullo P, Di Meo S, Agnisola C (1999) Protection Against Ischamia-Reperfusion Induced Oxidative Stress by Vitamin E Treatment. Arch. Physiol. Biochem. 107 (1): 27–34. DOI: https://doi.org/10.1076/apab.107.1.27.4355.
65. Tan DX, et al. (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2 (2): 181–197.
66. Voet D, Voet JG (2011) Biochemistry (J. Wiley & Sons) Available at: https://books.google.co.in/books/about/Biochemistry_4th_Edition.html?id=ne0bAAAAQBAJ.
67. Armiger LC, Clare L (1973) An Investigation of the rôle of lactic acid in myocardial infarction. Available at: https://researchspace.auckland.ac.nz/handle/2292/3143. https://researchspace.auckland.ac.nz/handle/2292/3143
68. Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 17 (12). doi:10.3390/ijms17122124. DOI: https://doi.org/10.3390/ijms17122124.
69. He C, et al. (2016) Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 17 (6). doi:10.3390/ijms17060939. https://doi.org/10.3390/ijms17060939.
70. Tousoulis D, Oikonomou E, Economou EK, Crea F, Kaski JC (2016) Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur. Heart J. 37 (22): 1723–1732. DOI: https://doi.org/10.1093/eurheartj/ehv759.
71. Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 83 (2): 456S–460S. DOI: https://doi.org/10.1093/ajcn/83.2.456S.
72. Khaper N, et al. (2010) Targeting the vicious inflammation–oxidative stress cycle for the management of heart failure. Antioxid. Redox. Signal 13 (7): 1033–1049. DOI: https://doi.org/10.1089/ars.2009.2930.
73. Esposito E, Cuzzocrea S (2010) Antiinflammatory activity of melatonin in central nervous system. Curr. Neuropharmacol. 8 (3): 228–42. DOI: 10.2174/157015910792246155.
74. Veneroso C, Tuñón MJ, González-Gallego J, Collado PS (2009) Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal Res. 47 (2):184–191. DOI: https://doi.org/10.1111/j.1600-079X.2009.00699.x.
75. Favero G, Franceschetti L, Bonomini F, Rodella LF, Rezzani R (2017) Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017: 1835195. DOI: https://doi.org/10.1155/2017/1835195.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


