Adrenaline induced disruption of endogenous melatoninergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study
Adrenaline disrupts melatonergic and antioxidant system
Abstract
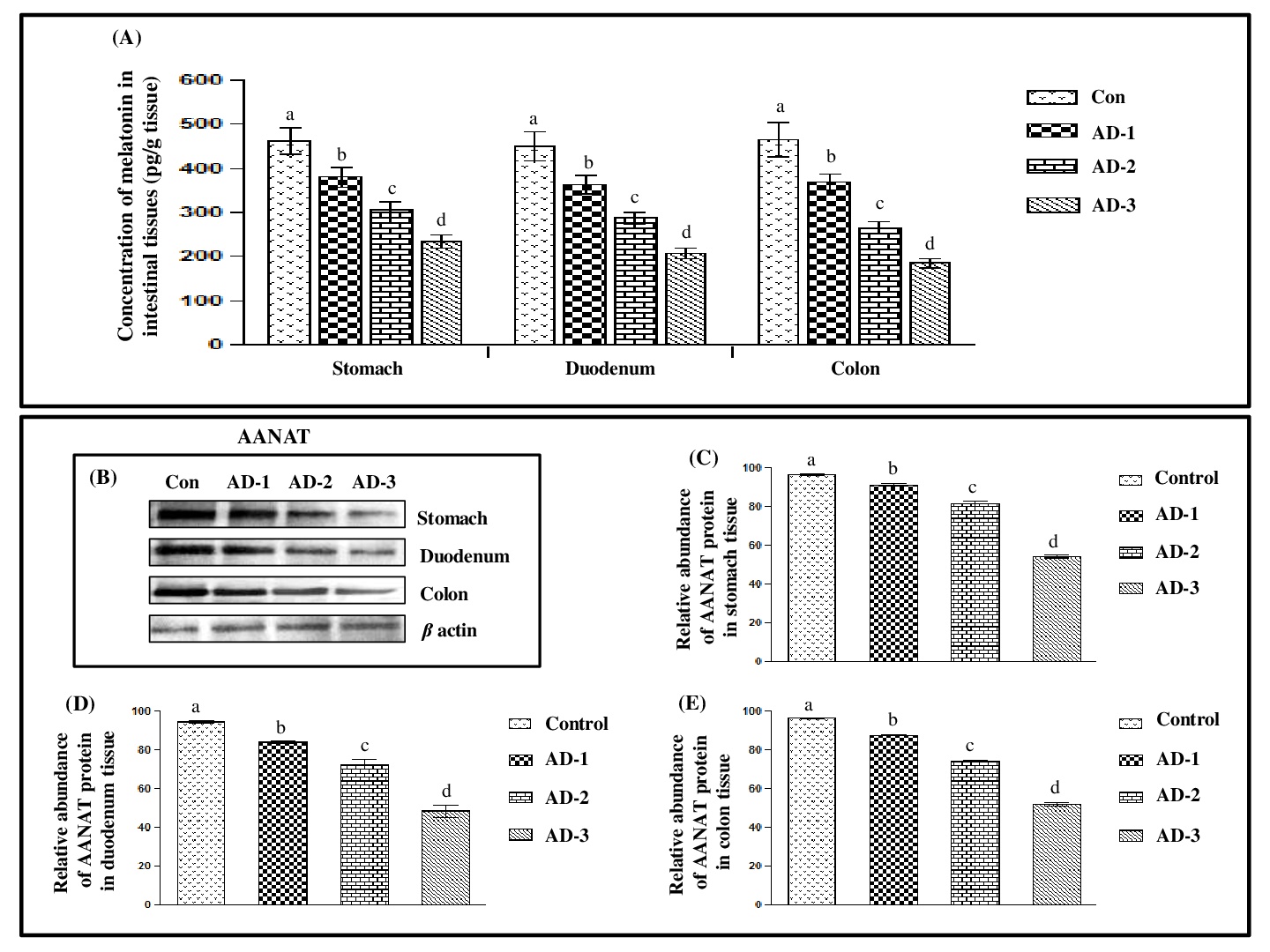
The current study aimed to demonstrate the potentially adverse effects of adrenaline, an endogenous stressor, on the melatonergic system, oxidative status, antioxidative responses and inflammatory markers in different parts of gastrointestinal tract of Wistar rat. These included stomach, duodenum and colon and they were incubated with different concentrations (2.5, 5.0 and 10.0 µg/mL) of adrenaline for 1h, respectively. The levels of melatonin, gene expressions of arylalkylamine N-acetyltransferase (AANAT) and melatonin receptor 1 (MT1) as well as other stress-induced parameters including NF-kB expression, levels of cAMP, calcium, malondialdehyde, protein carbonyl content, reduced glutathione, nitrate, superoxide dismutase, catalase, glutathione peroxidase and glutathione S-transferase, tumour necrosis factor-α, IL-1β, IL6 and IL10 were systemically measured in these tissues. An adrenaline dose-dependent decrease in level of melatonin, AANAT, MT1 and NF-kB in these tissues were observed. In contrast, the profound increases in the levels of cAMP, calcium and all oxidative stress markers, inflammatory cytokines (except IL10), and activities of antioxidant enzymes (except superoxide dismutase) were observed after adrenaline treatment. A maximum effect was found in tissues treated with 5 µg/mL of adrenaline. The Correlation studies between melatonin level and other parameters (any two at a time) indicated a potentially physiological interplay between adrenaline stress and melatonin tissue levels. Collectively, the results provided the novel data on the adverse effects of adrenaline on the endogenous melatonergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of rats.
References
2. Cahova M, Palenickova E, Papackova Z, Dankova H, Skop V, Kazdova L (2012) Epinephrine-dependent control of glucose metabolism in white adipose tissue: The role of α- and β-adrenergic signalling. Exp. Biol. Med. 237: 211−218. DOI: 10.1258/ebm.2011. 011189.
3. Gavrilovic L-J, Stojiljkovic V, Kasapovic J, Pejic S, Todorovic A, Pajović B-S, Dronjak S (2012) Chronic physical stress changes gene expression of catecholamine biosynthetic enzymes in the adrenal medulla of adult rats. Acta Vet-Beograd. 62: 151–169. DOI: 10.2478/acve-2014-0027.
4. Flint M-S, Baum A, Chambers W-H, Jenkins F-J (2007) Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psycho. Neuroendocrinol. 32: 470–479. DOI: 10.1186/bcr3189.
5. Costa V-M, Silva R, Ferreira L-M, Branco P-S, Carvalho F, Bastos M-L, Carvalho R-A, Carvalho M, Remião F (2007) Oxidation process of adrenaline in freshly isolated rat cardiomyocytes: formation of adrenochrome, quinoproteins, and GSH adduct. Chem. Res. Toxicol. 20: 1183–1191. DOI: 10.1021/tx7000916.
6. Rudra S, Mukherjee D, Dutta M, Ghosh A-K, Dey M, Basu A, Pattari S-K, Chattopadhyay A, Bandyopadhyay D (2014) Orally administered melatonin protects against adrenaline-induced oxidative stress in rat liver and heart: Involvement of antioxidant mechanism(s). J. Pharm. Res. 8: 303–320. http://jprsolutions.info/files/final-file-56bff9adee9ad8.28733292.pdf
7. Liu X, Wu W-K, Yu L, Sung J-J, Srivastava G, Zhang S-T, Cho C-H (2008) Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J. Cell Biochem.105: 53–60. DOI: 10.1002/jcb.21802.
8. Yao H, Duan Z, Wang M, Awonuga A-O, Rappolee D, Xie Y (2009) Adrenaline induces chemoresistance in HT-29 colon adenocarcinoma cells. Cancer Genet. Cytogenet.190: 81–87. DOI: 10.1016/j.cancergencyto.2008.12.009.
9. Andrea Č, Lada Ž, Dijana Ž, Ninoslav D, Vladan B, DraganaD, Biljana S-P (2014) Protective effect of dry olive leaf extract in adrenaline induced DNA damage evaluated using in vitro comet assay with human peripheral leukocytes. Toxicol. in Vitro 28: 451–456. DOI: 10.1016/j.tiv.2013.12.014.
10. Radaković M, Delić N, Stevanović J, Anđelković M, Kolarević S, Dačić S, Stanimirović Z (2014) The investigation of DNA damage induced by adrenaline in human lymphocytes in vitro. Acta Vet. Beograd. 64: 281–292. DOI: 10.2478/acve-2014-0027.
11. Wuren M, Yougang Z, Huangrong L, Banchao S, Jiefeng L, Jin Y, Changrong W, Xiaolong X, Yuying H, Xiaoxi L, Fenghua L, Jianqin X (2013) Effect of transport stress on peripheral blood lymphocyte subsets and the cytokines in pigs. Acta Vet-Beograd. 63: 177–190. DOI: 10.2298/AVB1303177W.
12. Raugstad T-S, Svanes K, Ulven A, Molster A (1979) Interaction between acute gastric ulcer and epinephrine-induced mucosal erosions in the rat: the significance of gastric acid secretion. Digestion. 19: 70–72. DOI: 10.1159/000198325.
13. Yildirim A, Sahin Y-N, Suleyman H, Yilmaz A, Yildrim S (2007) The role of prednisolone and epinephrine on gastric tissue and erythrocyte antioxidant status in adrenalectomized rats. J. Physiol. Pharmacol. 58: 105–116. http://www.jpp.krakow.pl/ journal/archive/03_07/pdf/ 105_03 _ 07_article. Pdf.
14. Tan D-X, Chen L-D, Poeggeler B, Manchester L-C, Reiter R-J (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr.J. 1: 57–60.
15. Bandyopadhyay D, Chattopadhyay A (2006) Reactive oxygen species-induced gastric ulceration: protection by melatonin. Curr. Med. Chem. 13: 1187–1202. DOI: 10.2174/ 092986706776360842.
16. Maitra S-K, Pal P-K (2017) Structural diversity and functional integrity of the fish pineal gland. In: Catalá A. (Ed.) Pineal gland: Research advances and clinical challenges. Nova Science Publishers, Inc. New York, USA. Pp. 51–92. https://www.novapublishers.com/ catalog/product_ info.php? products_id=62459.
17. Lee P-P-N, Pang S-F (1993) Melatonin and Its Receptors in the Gastrointestinal Tract. Neurosignals 2: 181–193. DOI: 10.1159/000109491.
18. Pal P-K, Hasan K-N, Maitra S-K (2016) Gut melatonin response to microbial infection in carp Catlacatla. Fish Physiol. Biochem. 42: 579–592. DOI: 10.1007/s10695-015-0161-7.
19. Pal P-K, Maitra S-K (2018) Response of gastrointestinal melatonin, antioxidants, and digestive enzymes to altered feeding conditions in carp (Catlacatla). Fish Physiol. Biochem. 44: 1061–1073. https://doi.org/10.1007/s10695-018-0494-0.
20. Rodriguez C, Mayo J-C, Sainz R-M, Antolin I, Herrera F, Martin V, Reiter R-J (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36: 1–9. https://doi.org/10.1046/j.1600-079X.2003.00092.
21. Reiter R-J, Tan D-X, Lorena F-B (2010) Melatonin: a multitasking molecule. Prog. Brain Res. 181: 127–151. DOI: 10.1016/S0079-6123(08)81008-4.
22. Trivedi P-P, Jena G-B (2013) Melatonin reduces ulcerative colitis- associated local and systemic damage in mice: investigation on possible mechanisms. Dig. Dis. Sci. 58: 3460–3474.doi: 10.1007/s10620-013-2831-6.
23. Kvetnoy I-M, Ingel I-E, Kvetnaia T-V, Malinovskaya N-K (2002) Gas¬trointestinal melatonin: cellular identification and biological role. Neuro Endocrinol Lett23, 121–132.
24. Chen C-Q, Fichna J, Bashashati M, Li Y-Y, Storr M (2011) Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 17: 3888–3898. DOI: 10.3748/wjg.v17.i34.3888.
25. Klein D (2007) ArylalkylamineN-acetyltransferase: “the Timezyme”. J. Biol. Chem. 282: 4233–4237. DOI: 10.1074/jbc.R600036200.
26. Chowdhury I, Maitra S-K (2012) Melatonin in the promotion of health. In: Watson RR, editor. Melatonin time line: from discovery to therapy. Boca Raton (FL): Taylor and Francis; Pp. 1–60.
27. Pal P-K, Maitra S-K (2017) Neuronal control of gut melatoninergic system in carp. BAOJ Neuro. 2: 024. https://bioaccent.org/neurology/neurology24.pdf.
28. Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter R-J (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36: 195–203. https://doi.org/ 10.1111/j.1600-079X.2004.00118.x.
29. Laemmli U-K (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 27: 680–685.
30. Draper H-H, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 186: 421–431. https://doi.org/10.1016/00766879 (90)86135-I.
31. Levine R-L, Williams J-A, Stadtman E-R, Shacter E (1994) Carbonyl assays for determination of oxidatively modified. Meth. Enzymol. 233: 346–357. https://doi.org/ 10.1016 /S0076-6879(94)33040-9.
32. Green L-C, Wagner D-A, Glogowski J, Skipper P-L, Wishnok J-S, Tannenbaum S-R (1982) Analysis of nitrate, nitrite, and (15N) nitrate in biological fluids. Anal Biochem. 126: 131–138. https://doi.org/10.1016/0003-2697(82)90118-X.
33. Ewing J-F, Janero D-R (1995) Microplate superoxide dismutase assay employing a non-enzymatic superoxide generator. Anal Biochem. 232: 243–248. DOI: 10.1006/abio.1995. 0014.
34. Aebi H (1984) Catalase in vitro. Methods Enzymol. 105: 121–126. https://doi.org/10.1016/ S0076-6879(84)05016-3.
35. Aksoy Y, Balk M, Öğüs I-H, Özer N (2004) The mechanism of inhibition of human erythrocyte catalase by azide. Turk. J. Biol. 28: 65–70. https://pdfs.semanticscholar.org /2e5f/7 4bdb8945b7b3ceb8569abdee451c9a12a79.pdf.
36. Castro R, Piazzon M-C, Noya M, Leiro J-M, Lamas J (2008) Isolation and molecular cloning of a fish myeloperoxidase. Mol Immunol45, 428–437. DOI: 10.1016/j. molimm.2007.05.028
37. Habig W-H, Pabst M-J, Jakoby W-B (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249: 7130–7139.
38. Pinto R-E, Bartley W (1969) The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem. J. 115: 865.b1. DOI: 10.1042/bj1150865a.
39. Ellman G-L (1959) Tissue sulfhydryl groups. Arch Biochem. Biophys. 82: 70–77.
40. Lowry, O-H, Rosebrough N-J, Farr A-L, Randall R-J (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
41. Zar J-H (1999) Biostatistical analysis. 4th ed. Upper Saddle River (NJ), Prentice Hall.
42. Parlakpinar H, Orum M-H, Sagir M (2013) Pathophysiology of myocardial ischemia reperfusion injury: a review. Med. Sci. 2: 935–954. DOI: 10.5455/medscience.2013.02.8082.
43. Carpagnano G-E, Kharitonov S-A, Resta O (2003) 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 124: 1386–1392.
44. Kwiecien S, Brzozowski T, Konturek PC (2004) Gastroprotection by pentoxyfilline against stress-induced gastric damage. Role of lipid peroxidation, antioxidizing enzymes and proinflammatory cytokines. J. Physiol. Pharmacol. 55: 337–355. http://www.jpp. krakow.pl/journal/archive /06_04/pdf/337_06_04_article.pdf.
45. Singal P-K, Kapur N, Dhillon K-S, Beamish R-E, Dhalla N-S (1981) Role of free radicals in catecholamine-induced cardiomyopathy. Can. J. Physiol. Pharmacol. 60: 390–397.
46. Noronha Dutra A-A, Steen E-M (1982) Lipid peroxidation as a mechanism of injury in cardiac myocytes. Lab. Invest. 47: 346–353.
47. Sandra J-P, Devid J-D, Arend B, lawrence L-S (1998) Effects of epinephrine on lipid metabolism in resting skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 913: 1849–1893. https://doi.org/10.1152/ajpendo.1998.275.2.E300.
48. Isabella D-D, Rossi R, Giustarini D, Milzani A, Colombo R(2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 329: 23–38. https://doi.org/ 10.1016 /S0009-8981(03)00003-2.
49. Luiz F, Costa Rosa B-P, Danilo A-S, Yara C, Rui C (1992) Effect of epinephrine on glucose metabolism and hydrogen peroxide content in incubated rat macrophages. Biochem. Pharmacol. 44: 2235–2241. https://doi.org/10.1016/0006-2952(92)90352-J.
50. D’atril P, Malaver E, Romaniuk M-A, Pozner R-G, Negrotto S, Schattner M (2009) Nitric oxide: news from stem cells to platelets. Curr. Med. Chem. 16: 417–429. http://dx.doi.org/ 10.2174/092986709787315513.
51. Ugochukw U-N-H, Babady N-E, Cobourne M, Gasset S-R (2003) The effect of Gongronema latifolium extracts on serum lipid profile and oxidative stress in hepatocytes of diabetic rats. J. Biosci. 28: 1–5.
52. Dobrzynska, M-M, Baumgartner A, Anderson D (2004) Antioxidants modulate thyroid hormone and noradrenaline-induced DNA damage in human sperm. Mutagenesis 19: 325–330. https://doi.org/10.1093/mutage/geh037.
53. Guo Y, Sun J, Li T, Zhang Q, Bu S, Wang Q, Lai D-M (2017) Melatonin ameliorates restrain stress-induced oxidative stress and apoptosis in testicular cells via NF-kβ/iNOS and Nrf2/HO-1 signaling pathway. Sci. Rep. 7: 9599. DOI: 10.1038/s41598-017-09943-2.
54. Williamson J-R, Schaffer S (1976) Epinephrine, cyclic AMP, calcium, and myocardial contractility. Recent. Adv. Stud. Cardiac. Struct. Metab. 9: 205–223.
55. Delgado M, Fernández-Alfonso M-S, Fuentes A (2002) Effect of adrenaline and glucocorticoids on monocyte cAMP-specific phosphodiesterase (PDE4) in a monocytic cell line. Arch. Dermatol. Res. 294: 190–197. DOI 10.1007/s00403-002-0313-3.
56. Buetler T-M, Krauskopf A, Ruegg U-T (2004) Role of superoxide as a signaling molecule. News Physiol. Sci. 19: 120–123. DOI: 10.1152/nips.01514.2003.
57. Rikans L-E, Hornbrook K-R (1997) Lipid peroxidation, antioxidant protection and aging. Biochim. Biophys. Acta 1362: 116–127. https://doi.org/10.1016/S0925-4439(97)00067-7.
58. Bukan N, Sancak B, Yavuz O, Koca C, Tutkun F, Ozcelikay A-T, Altan N (2003) Lipid peroxidation and scavenging enzyme levels in the liver of streptozotocin-induced diabetic rats. Indian J. Biochem. Biophy. 40: 447–450.
59. Maisel A-S, Murray D-I-M, Rearden A, Irwin M, Michel M-C (1991) Propanolol treatment affects parameters of human immunity. Immunopharmacol. 22: 157–164. https://doi.org /10.1016/0162-3109(91)90040-6.
60. Talero E, Sánchez-Fidalgo S, Alarcán de la Lastra C, Illanes M, Calvo J-R, Motilva V (2008) Acute and chronic responses associated with adrenomedullin administration in experimental colitis. Peptides 29: 2001–2012. DOI:10.1016/j.peptides.2008.07.013.
61. Li B, Zani A, Martin Z, Lee C, Zani-Ruttenstock E, Eaton S, Pierro A. (2016) Intestinal epithelial cell injury is rescued by hydrogen sulphide. J Pediatr. Surg. 51: 775–778. DOI: 10.1016/j.jpedsurg.2016.02.019.
62. Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P, Zhang J-H, Sun X, Yuan H (2009) Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic. Res. 43: 478–484. DOI: 10.1080/10715760902870603.
63. Bekyarova G, Tzaneva M (2015) Melatonin ameliorates burn-induced liver injury by modulation of Nrf2 and NF-kB signalling pathways. SOJ Immunology 3: 1–8. DOI: http://dx.doi.org/10.15226/soji/3/3/00128.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


