Amelioration of adrenaline induced oxidative gastrointestinal damages in rat by melatonin through SIRT1-NFκB and PGC1α-AMPKα cascades
Melatonin prevents adrenaline induced intestinal damage
Abstract
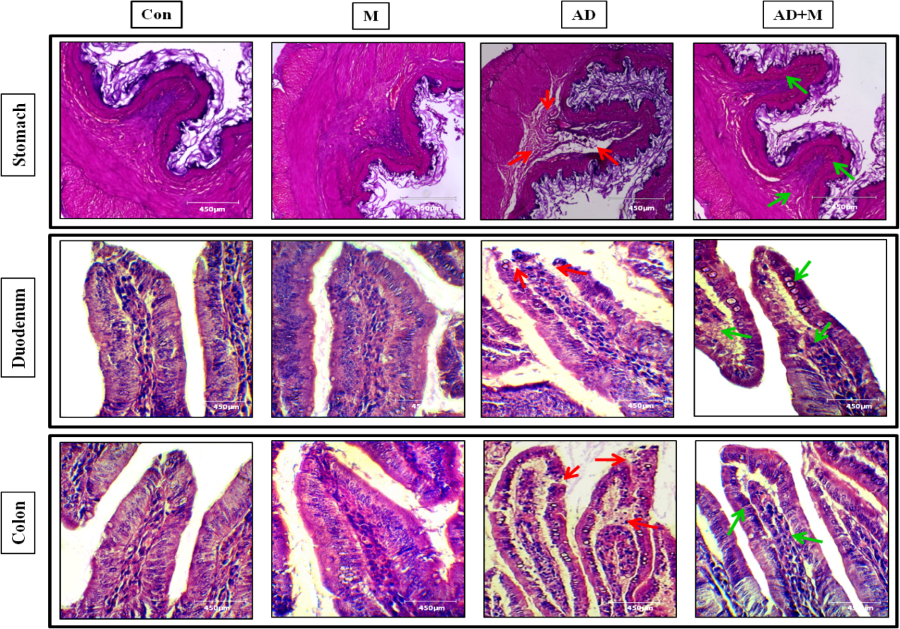
Adrenaline at high pharmacological doses may lead to oxidative damages in diverse organs including gut. In this study, we attempt to elucidate the potentially protective effects of melatonin on gastrointestinal (GI) tissue damages induced by adrenaline. Rats were injected (s.c.) with different doses (0.125, 0.25 and 0.50 mg/kg) of adrenaline bitartrate (AD) for 15 days with or without melatonin (2.5, 5 and 10 mg/kg; orally). The results showed that adrenaline caused massive histological and ultra-structural GI injuries and melatonin (20 mg/kg) effectively protected these injuries. The protective mechanisms are related to the antioxidant and anti-inflammatory activities of melatonin indicated by increased glutathione levels and antioxidant enzymes as well as decreased oxidative stress markers and pro-inflammatory cytokines in GI tissues. The signal pathways of melatonin include up-regulating expression of Nrf2, SIRT1 and Bcl2, while down-regulating NFκB, TNFα and Bax. Melatonin also targeted mitochondrial energy homeostasis and biogenesis by up-regulating expression of PGC1α, AMPKα and SOD2 and reduced leakage of cytochrome c. The SIRT1-NFκB and PGC1α-AMPKα signal transduction pathways seem to play the central roles involving in melatonin’s protective effects on gastric damages induced by the high doses of adrenaline.
References
2. Gavrilovic LJ, Stojiljkovic V, Kasapovic J, Snežana P, Ana TP, Snežana B, Slađana D (2012) Chronic physical stress changes gene expression of catecholamine biosynthetic enzymes in the adrenal medulla of adult rats. Acta Vet-Beograd. 62 (2-3): 151–169. DOI: 10.2478/acve-2014-0027.
3. Kemp SF, Lockey RF, Simons FE (2008) World Allergy Organization ad hoc Committee on epinephrine in anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 63 (8): 1061-1070. DOI: 10.1111/j.1398-9995.2008.01733.x.
4. Kinsella SM, Tuckey JP (2001) Perioperative bradycardia and asystole: relationship to vasovagal syncope and the Bezold–Jarisch reflex. Br. J. Anaesth. 86 (6): 859-868. DOI: 10.1093/bja/86.6.859.
5. Numa AH, Williams GD, Dakin CJ (2001) The effect of nebulized epinephrine on respiratory mechanics and gas exchange in bronchiolitis. Am. J. Resp. Crit. Care 164 (1): 86-91. DOI: 10.1164/ajrccm.164.1.2008090.
6. Reiter PD, Roth J, Wathen B, LaVelle J, Ridall LA (2018) Low-dose epinephrine boluses for acute hypotension in the PICU. Pediatr. Crit. Care Med. 19 (4): 281-286. DOI: 10.1097/PCC. 0000000000001448.
7. Costa VM, Silva R, Ferreira LM, Branco PS, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F (2007) Oxidation process of adrenaline in freshly isolated rat cardiomyocytes: formation of adrenochrome, quinoproteins, and GSH adduct. Chem. Res. Toxicol. 20 (8): 1183-1191. DOI: 10.1021/tx7000916.
8. Rudra S, Mukherjee D, Dutta M, Ghosh AK, Dey M, Basu A, Pattari SK, Chattopadhyay A, Bandyopadhyay D (2014) Orally administered melatonin protects against adrenaline-induced oxidative stress in rat liver and heart: Involvement of antioxidant mechanism (s). J. Pharm. Res. 8: 303-320. http://jprsolutions.info/files/final-file-56bff9adee9ad8. 28733292.pdf.
9. Pal PK, Bhattacharjee B, Ghosh AK, Chattopadhyay A, Bandyopadhyay D (2018) Adrenaline induced disruption of endogenous melatoninergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Res. 1 (1): 109-131. DOI: https://doi.org/https://doi.org/10.32794/ mr11250007.
10. Djelić N, Radaković M, Borozan S, Dimirijević-Srećković V, Pajović N, Vejnović B, Borozan N, Bankoglu EE, Stopper H, Stanimirović Z (2019) Oxidative stress and DNA damage in peripheral blood mononuclear cells from normal, obese, prediabetic and diabetic persons exposed to adrenaline in vitro. Mutat. Res-Gen. Tox. En. 843: 81-89. https://doi.org/10.1016/j.mrgentox.2019.01.013.
11. Korać J, Stanković DM, Stanić M, Bajuk-Bogdanović D, Žižić M, Pristov JB, Grgurić-Šipka S, Popović-Bijelić A, Spasojević I (2018) Coordinate and redox interactions of epinephrine with ferric and ferrous iron at physiological pH. Sci. Rep. 8 (1): 3530. DOI: 10.1038/s41598-018-21940-7.
12. Flint MS, Baum A, Chambers WH, Jenkins FJ (2007) Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinol. 32 (5): 470–479. DOI: 10.1016/j.psyneuen.2007.02.013.
13. Romeo F, Li D, Shi M, Mehta JL (2000) Carvedilol prevents epinephrine-induced apoptosis in human coronary artery endothelial cells: modulation of Fas/Fas ligand and caspase-3 pathway. Cardiovasc. Res. 45 (3): 788-794. DOI: 10.1016/s0008-6363(99) 00369-7.
14. Liu X, Wu WK, Yu L, Sung JJ, Srivastava G, Zhang ST, Cho CH (2008) Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta adrenoceptor dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. Cell. Biochem. 105 (1): 53-60. DOI: 10.1002/jcb.21802.
15. Yao H, Duan Z, Wang M, Awonuga AO, Rappolee D, Xie Y (2009) Adrenaline induces chemoresistance in HT-29 colon adenocarcinoma cells. Cancer Genet. Cytogenet. 190 (2): 81-87. DOI: 10.1016/j.cancergencyto.2008.12.009.
16. Raugstad TS, Svanes K, Ulven A, Molster A (1979) Interaction between acute gastric ulcer and epinephrine-induced mucosal erosions in the rat: the significance of gastric acid secretion. Digestion 19: 70–72. DOI: 10.1159/000198325.
17. Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1: 57–60. DOI: 10.1111/j.1600-079x.1993.tb00498.x.
18. Periasamy S, Lee BF, Hsu DZ, Liu MY (2019) Melatonin aggravated oxaliplatin-mimicking sinusoidal obstruction syndrome: role of platelet aggregation and oxidative stress. OBM Neurobiol. 3 (3): 22. DOI: 10.21926/obm.neurobiol.1903033.
19. Huether G (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49 (8): 665–670. DOI: 10.1007/bf01923948.
20. Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019a) Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res. 2 (2): 158-184. DOI: 10.32794/mr11250027.
21. Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36 (1):1–9. https://doi.org/10.1046/ j.1600-079X.2003.00092.
22. Pal PK, Sarkar S, Chattopadhyay A, Bandyopadhyay D (2019b) Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals. Melatonin Res. 2(4):61-82. DOI: 10.32794/mr11250041.
23. Chowdhury I, Maitra SK (2012) Melatonin in the promotion of health. In: Melatonin time line: from discovery to therapy. Watson RR (Ed.) Boca Raton (FL): Taylor and Francis. Pp. 1-60.
24. Bubenik GA, Niles LP, Pang SF, Pentney PJ (1993) Diurnal variation and binding characteristics of melatonin in the mouse brain and gastrointestinal tissues. Comp. Biochem. Physiol. 104: 221–224. DOI: 10.1016/0742-8413(93)90027-i.
25. Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36 (3): 195-203. https://doi.org/ 10.1111/j.1600-079X.2004.00118.x.
26. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. DOI: 10.1038/227680a0.
27. Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 186: 421-431. https://doi.org/10.1016/00766879 (90)86135-I.
28. Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified. Methods Enzymol. 233: 346-357. https://doi.org/ 10.1016 /S0076-6879(94)33040-9.
29. Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem. Biophys. 82: 7077. DOI: 10.1016/0003-9861(59)90090-6.
30. Ewing JF, Janero DR (1995) Microplate superoxide dismutase assay employing a non-enzymatic superoxide generator. Anal. Biochem. 232 (2): 243-248. DOI: 10.1006/abio.1995. 0014.
31. Aebi H (1984) Catalase in vitro. Methods Enzymol. 105: 121-126. https://doi.org/10.1016/ S0076-6879(84)05016-3.
32. Castro R, Piazzon MC, Noya M, Leiro JM, Lamas J (2008) Isolation and molecular cloning of a fish myeloperoxidase. Mol. Immunol. 45 (2): 428-437. DOI: 10.1016/j. molimm.2007.05.028.
33. Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249 (22): 7130-7139.
34. Pinto RE, Bartley W (1969) The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem. J. 112 (1):109-115. DOI: 10.1042/bj1150865a.
35. Roy SG, De P, Mukherjee D, Chander V, Konar A, Bandyopadhyay D, Bandyopadhyay A (2009) Excess of glucocorticoid induces cardiac dysfunction via activating angiotensin II pathway. Cell Physiol. Biochem. 24 (1-2): 1-10.DOI: 10.1159/000227803.
36. Pal PK, Hasan KN, Maitra SK (2016) Gut melatonin response to microbial infection in carp Catla catla. Fish Physiol. Biochem. 42 (2): 579-592. DOI: 10.1007/s10695-015-0161-7.
37. MohanKumar K, killingsworth CR, McLwaim RB, Timpa JG, Jagadeeswaran R, Namachivayam K, Kurundkar AR, Kelly DR, Garzon SA, Maheshwari A (2014) Intestinal epithelial apoptosis initiates gut mucosal injury during extracorporeal membrane oxygenation in the newborn piglet. Lab. Invest. 94: 150-160. DOI: 10.1038/labinvest.2013.149.
38. Cheema KJ, Scofield AM (1982) Scanning electron microscopy of the intestines of rats infected with Nippostrongylus brasiliensis. Int. J. Parasitol. 12: 199-205. DOI: 10.1016/0020-7519(82)90017-0.
39. Mukherjee D, Ghosh AK, Bandyopadhyay A, Basu A, Datta S, Pattari SK, Reiter RJ, Bandyopadhyay D (2012) Melatonin protects against isoproterenol-induced alterations in cardiac mitochondrial energy-metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 53: 166–179. DOI:10.1111/j.1600-079X.2012.00984.x
40. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265-275.
41. Zar JH (1999) Biostatistical analysis, 4th Edn. Prentice Hall, Upper Saddle River.
42. Parlakpinar H, Orum MH, Sagir M (2013) Pathophysiology of myocardial ischemia reperfusion injury: a review. Med. Sci. 2 (4): 935-954. DOI: 10.5455/medscience.2013. 02.8082.
43. Yildirim A, Sahin YN, Suleyman H, Yilmaz A, Yildrim S (2007) The role of prednisolone and epinephrine on gastric tissue and erythrocyte antioxidant status in adrenalectomized rats. J. Physiol. Pharmacol. 58: 105-116.
44. Radaković M, Borozan S, Djelić N, Ivanović S, Miladinović DC, Ristanić M, Spremo-Potparević B, Stanimirović Z (2018) Nitroso-oxidative stress, acute phase response, and cytogenetic damage in Wistar rats treated with adrenaline. Oxid. Med. Cell Longev. 2018. Article ID 1805354. https://doi.org/10.1155/2018/1805354.
45. Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 329 (1-2): 23-38. https://doi.org/ 10.1016 /S0009-8981(03)00003-2.
46. Luiz F, Costa Rosa BP, Danilo AS, Yara C, Rui C (1992) Effect of epinephrine on glucose metabolism and hydrogen peroxide content in incubated rat macrophages. Biochem. Pharmacol. 44: 2235-2241. https://doi.org/10.1016/0006-2952(92)90352-J.
47. Ugochukw UNH, Babady NE, Cobourne M, Gasset SR (2003) The effect of Gongronema latifolium extracts on serum lipid profile and oxidative stress in hepatocytes of diabetic rats. J. Biosci. 28 (1): 1-5. DOI: 10.1007/bf02970124.
48. Dobrzynska MM, Baumgartner A, Anderson D (2004) Antioxidants modulate thyroid hormone and noradrenaline-induced DNA damage in human sperm. Mutagenesis 19 (4): 325-330. https://doi.org/10.1093/mutage/geh037.
49. Guo Y, Sun J, Li T, Zhang Q, Bu S, Wang Q, Lai DM (2017) Melatonin ameliorates restrain stress-induced oxidative stress and apoptosis in testicular cells via NFkβ/iNOS and Nrf2/HO-1 signaling pathway. Sci. Rep. 7 (1): 9599. DOI: 10.1038/s41598-017-09943-2.
50. DeRijk RH, Boelen A, Tilders FJ, Frank B (1994) Induction of plasma interleukin-6 by circulating adrenaline in the rat. Psychoneuroendocrinology 19 (2): 155-163. DOI: 10.1016/0306-4530(94)90005-1.
51. Chen S, Liu GL, Li MM, Liu R, Liu H (2017) Effects of epinephrine on inflammation-related gene expressions in cultured rat cardiomyocytes. Transl. Perioper. Pain Med. 2 (1): 13-19.
52. Ferreira ZS, Fernandes PA, Duma D, Assreuy J, Avellar MC, Markus RP (2005) Corticosterone modulates noradrenaline-induced melatonin synthesis through inhibition of nuclear factor kappa B. J. Pineal Res. 38 (3): 182-188. DOI: 10.1111/j.1600-079X.2004.00191.x.
53. Das N, Mandala A, Naaz S, Giri, Jain M, Bandyopadhyay D, Reiter RJ, Roy SS (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J. Pineal Res. 62 (4): e12404. DOI: 10.1111/jpi.12404.
54. Negi G, Kumar A, Sharma SS (2011) Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF‐κB and Nrf2 cascades. J. Pineal Res. 50 (2): 124-131. DOI: 10.1111/j.1600-079X.2010.00821.x.
55. Yi G, Li L, Luo M, He X, Zou Z, Gu Z, Su L (2017) Heat stress induces intestinal injury through lysosome- and mitochondria-dependent pathway in vivo and in vitro. Oncotarget 8 (25): 40741-40755. DOI: 10.18632/oncotarget.16580.
56. Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong A (2008) Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 76 (11): 1485-1489. DOI: 10.1016/j.bcp.2008.07.017.
57. Yang Y, Jiang S, Dong Y, Fan C, Zhao L, Yang X, Li J, Di S, Yue L, Liang G, Reiter RJ, Qu Y (2015) Melatonin prevents cell death and mitochondrial dysfunction via a SIRT 1‐dependent mechanism during ischemic‐stroke in mice. J. Pineal Res. 58 (1): 61-70. DOI: 10.1111/jpi.12193.
58. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF‐κB‐dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23 (12): 2369-2380. DOI: 10.1038/sj.emboj.7600244.
59. Liu D, Ma Z, Di S, Yang Y, Yang J, Xu L, Reiter RJ, Qiao S, Yuan J (2018) AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic. Biol. Med. 129: 59-72. DOI: 10.1016/j.freeradbiomed.2018.08.032.
60. Guo P, Pi H, Xu S, Zhang L, Li Y, Li M, Cao Z, Tian L, Xie J, Li R, He M, Lu Y, Liu C, Duan W, Yu Z, Zhou Z (2014) Melatonin improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol. Sci. 142 (1): 182-195. DOI: 10.1093/toxsci/kfu164.
61. Wang SJ, Zhao XH, Chen W, Bo N, Wang XJ, Chi ZF, Wu W (2015) Sirtuin 1 activation enhances the PGC-1α/mitochondrial antioxidant system pathway in status epilepticus. Mol. Med. Rep. 11 (1): 521-526. DOI: 10.3892/mmr.2014.2724.
62. Kasahara E, Lin LR, Ho YS, Reddy VN (2005) Manganese superoxide dismutase protects against oxidation-induced apoptosis in mouse retinal pigment epithelium: implications for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 46 (9): 3426-3434. DOI: 10.1167/iovs.05-0344.
63. Epperly MW, Sikora CA, DeFilippi SJ, Gretton JA, Zhan Q, Kufe DW, Greenberger JS (2002) Manganese superoxide dismutase (SOD2) inhibits radiation-induced apoptosis by stabilization of the mitochondrial membrane. Radiat. Res. 157 (5): 568-577. DOI: 10.1667/0033-7587(2002)157[0568:msdsir] 2.0.co;2.
64. Reiners Jr JJ, Caruso JA, Mathieu P, Chelladurai B, Kessel D (2002) Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 9 (9): 934-944. DOI: 10.1038/sj.cdd.4401048.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


